
What is the geometrical shape of $Xe{{O}_{3}}$?
A. Trigonal pyramidal
B. Planar triangular
C. Square planar
D. Tetrahedral
Answer
598.2k+ views
Hint: The shape of a molecule is determined by the location of the nuclei and its electrons. We should have an idea about the chemical bonding formation of the compound in order to predict the geometrical shape. Think about the electronic configuration of $Xe$.
Complete answer:
In the compound $Xe{{O}_{3}}$, we can see that the central atom i.e. $Xe$ has the electronic configuration of $[Kr]4{{d}^{10}}5{{s}^{2}}5{{s}^{6}}$. The outermost shell contains 8 electrons, 6 of them are used up while bonding with 3 oxygen atoms and the other 2 form a lone pair. So the $Xe$ atom requires 4 orbitals to accommodate the 3 bond pairs of electrons and 1 lone pair of electrons. The other oxygen and the xenon atom are arranged into a trigonal pyramidal shape due to the presence of the lone pair on $Xe$. The structure of the atom is as follows:
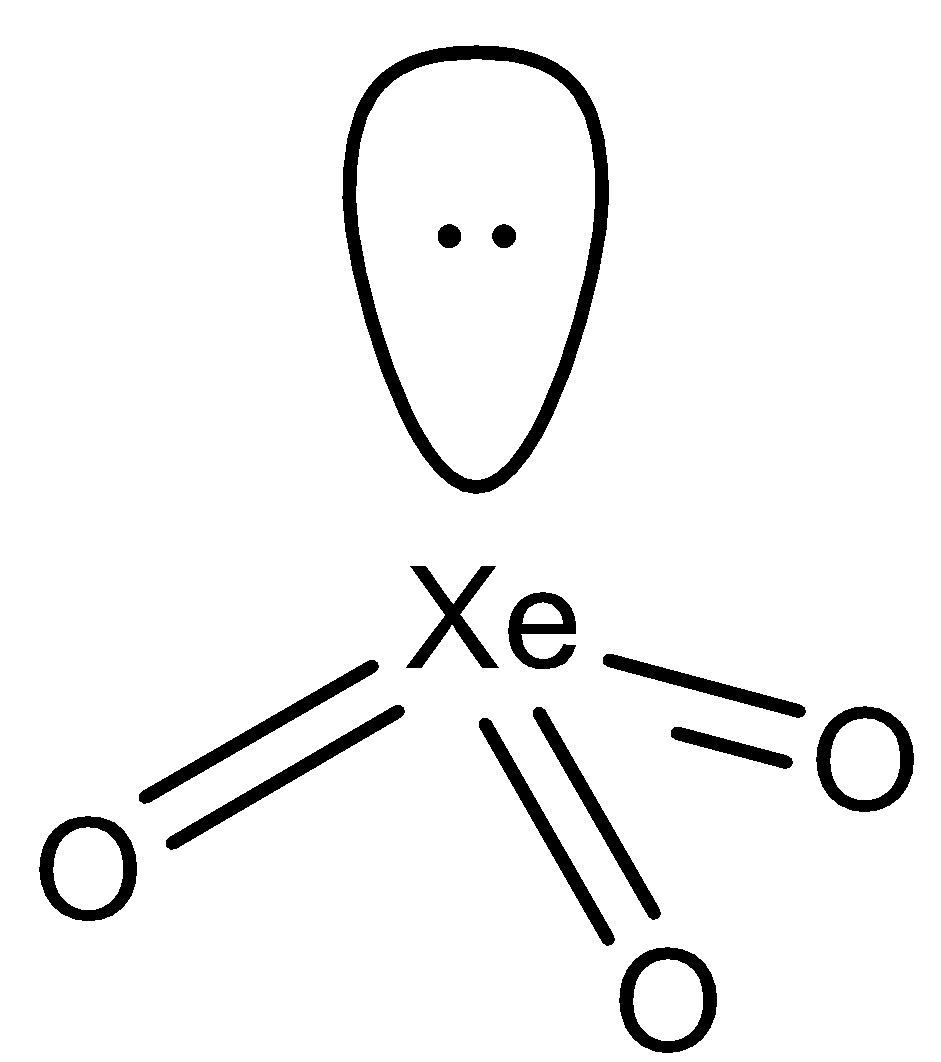
Hence, the answer to this question is ‘A. Trigonal pyramidal’
Additional Information:
In chemistry a trigonal pyramidal shape denotes a molecule geometry with one atom at the apex and three atoms at the corners of a triangle base, resembling a tetrahedron.
Note:
Note that the hybridization of the $Xe$ atom is $s{{p}^{3}}$ and thus has a tetrahedral shape. This tetrahedral shape is however, not seen as the geometry is considered to be pertaining only to the atom nuclei involved in the structure. So, the lone pair present is not considered to be a part of the geometry and the answer is trigonal pyramidal and not tetrahedral.
Complete answer:
In the compound $Xe{{O}_{3}}$, we can see that the central atom i.e. $Xe$ has the electronic configuration of $[Kr]4{{d}^{10}}5{{s}^{2}}5{{s}^{6}}$. The outermost shell contains 8 electrons, 6 of them are used up while bonding with 3 oxygen atoms and the other 2 form a lone pair. So the $Xe$ atom requires 4 orbitals to accommodate the 3 bond pairs of electrons and 1 lone pair of electrons. The other oxygen and the xenon atom are arranged into a trigonal pyramidal shape due to the presence of the lone pair on $Xe$. The structure of the atom is as follows:

Hence, the answer to this question is ‘A. Trigonal pyramidal’
Additional Information:
In chemistry a trigonal pyramidal shape denotes a molecule geometry with one atom at the apex and three atoms at the corners of a triangle base, resembling a tetrahedron.
Note:
Note that the hybridization of the $Xe$ atom is $s{{p}^{3}}$ and thus has a tetrahedral shape. This tetrahedral shape is however, not seen as the geometry is considered to be pertaining only to the atom nuclei involved in the structure. So, the lone pair present is not considered to be a part of the geometry and the answer is trigonal pyramidal and not tetrahedral.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




