
Why is geometrical isomerism not possible in tetrahedral complexes having two different types of unidentate ligands coordinated with the central metal ion?
Answer
595.5k+ views
Hint: Geometrical isomers can be basically explained as a phenomenon when two or more compounds have the same number of all constituent atoms, but all their geometric structures are different.
Complete Step-by-Step answer:
Tetrahedral complexes can be explained as the compounds in which the central atom of the compound is the centre for 4 other substituent atoms of the compound. The name of these complexes is derived for the tetrahedral geometries that these compounds have.
On the other hand, unidentate ligands are ligands that can donate a pair of electrons form any one atom of the ligand. Unidentate ligands are also known monodentate ligands.
When we consider a tetrahedral complex with two different types of unidentate ligands, we understand that due to the tetrahedral geometry of the structure, the relative positions of the unidentate ligands attached to the central atom are same with respect to each other. Because of this, geometrical isomerism not possible in tetrahedral complexes having two different types of unidentate ligands coordinated with the central metal ion
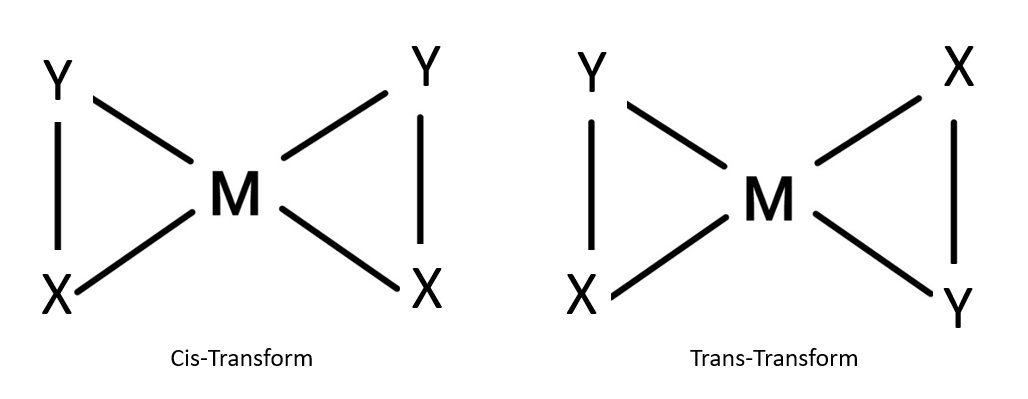
There are no other configurations possible other than these. Hence, we can diagrammatically represent why geometrical isomerism is not possible in tetrahedral complexes having two different types of unidentate ligands coordinated with the central metal ion where A and B are the two unidentate ligands and M is the central metal atom.
Note: Geometric isomers are possible for both square planar and octahedral complexes, but not tetrahedral because in tetrahedral all bond angles are equal. Optical isomers are possible for both tetrahedral and octahedral complexes, but not square planar.
Complete Step-by-Step answer:
Tetrahedral complexes can be explained as the compounds in which the central atom of the compound is the centre for 4 other substituent atoms of the compound. The name of these complexes is derived for the tetrahedral geometries that these compounds have.
On the other hand, unidentate ligands are ligands that can donate a pair of electrons form any one atom of the ligand. Unidentate ligands are also known monodentate ligands.
When we consider a tetrahedral complex with two different types of unidentate ligands, we understand that due to the tetrahedral geometry of the structure, the relative positions of the unidentate ligands attached to the central atom are same with respect to each other. Because of this, geometrical isomerism not possible in tetrahedral complexes having two different types of unidentate ligands coordinated with the central metal ion

There are no other configurations possible other than these. Hence, we can diagrammatically represent why geometrical isomerism is not possible in tetrahedral complexes having two different types of unidentate ligands coordinated with the central metal ion where A and B are the two unidentate ligands and M is the central metal atom.
Note: Geometric isomers are possible for both square planar and octahedral complexes, but not tetrahedral because in tetrahedral all bond angles are equal. Optical isomers are possible for both tetrahedral and octahedral complexes, but not square planar.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




