
What is the functional group of alcohol and phenol?
A) \[ - OH\] Phenol and
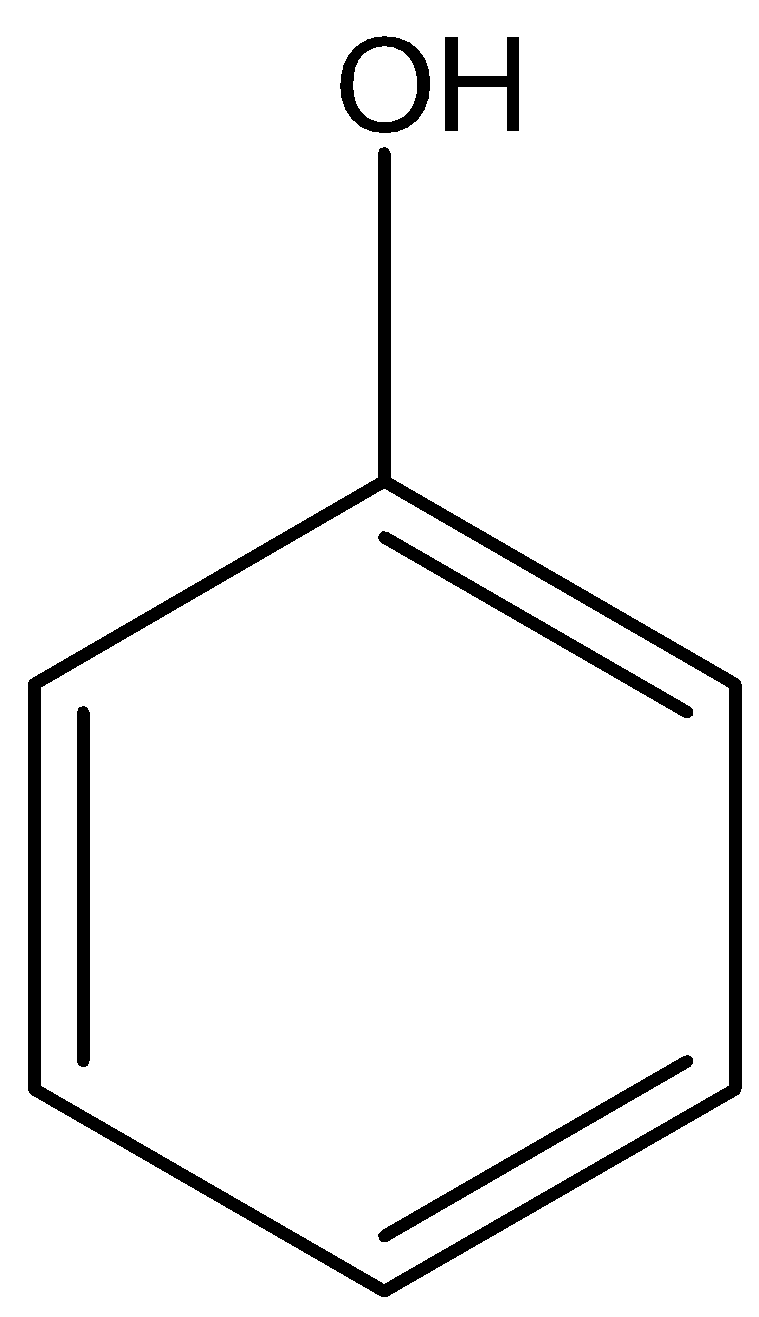 alcohol
alcohol
B)
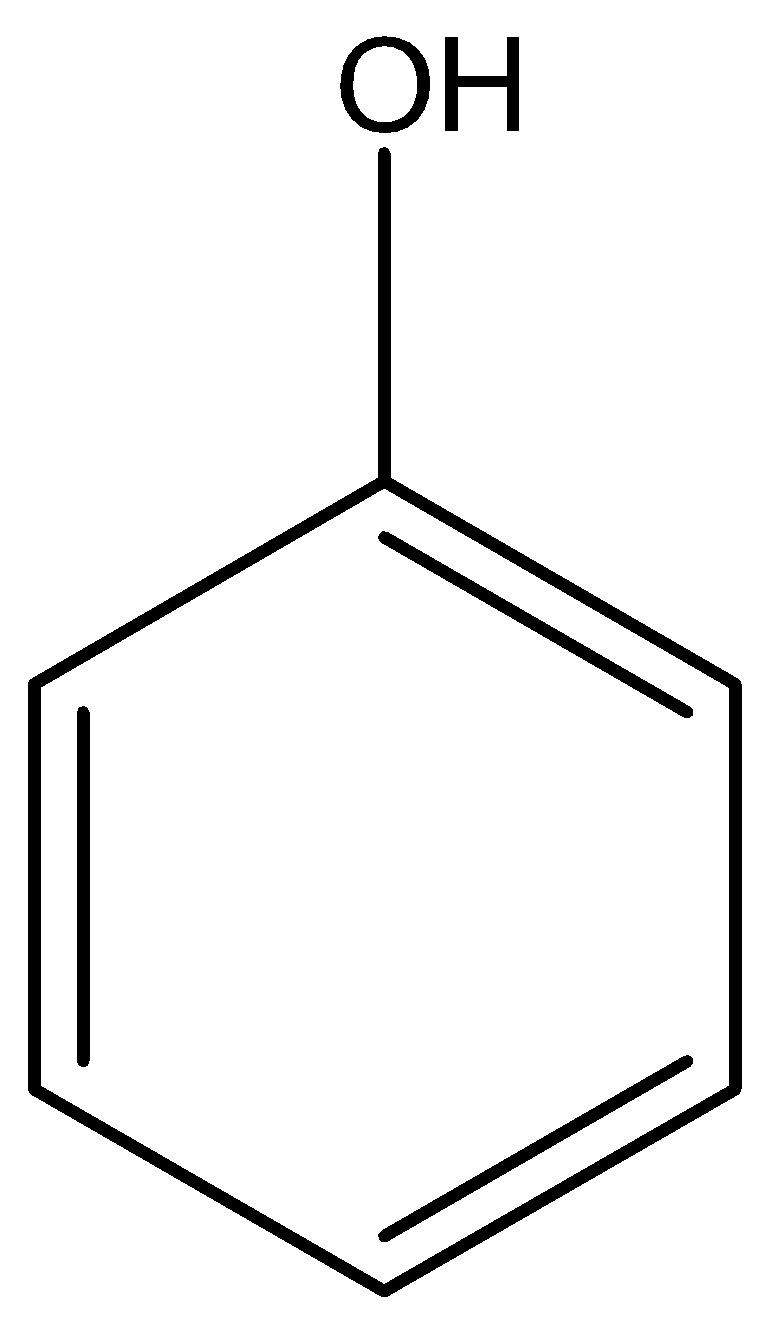 Phenol and \[ - OH\] alcohol
Phenol and \[ - OH\] alcohol
C) \[ - OH\] Alcohol and \[ - COOH\] Phenol
D) None of the above


Answer
524.1k+ views
Hint: The general formula of alcohol is \[{C_n}{H_{2n + 1}}OH\]. Alcohol and phenol is the two organic compounds containing the hydroxyl group i.e. \[ - OH\] in common.
We have to know that alcohol is an organic compound which has at least one hydroxyl group which is attached to the saturated carbon atom chain.
Complete step by step answer:
We have to remember that the nomenclature for writing the name of alcohol is Suffix “ol” is used when hydroxyl group is present as a higher priority in any compound whereas Prefix “hydroxy” is used when other groups are present as higher priority than alcohol.
Examples of alcohol:
\[{C_2}{H_5}OH\]- Ethanol
Phenol is an aromatic organic compound that contains hydroxyl group which is attached to a benzene ring therefore the functional group is \[-{C_6}{H_5}OH\]or
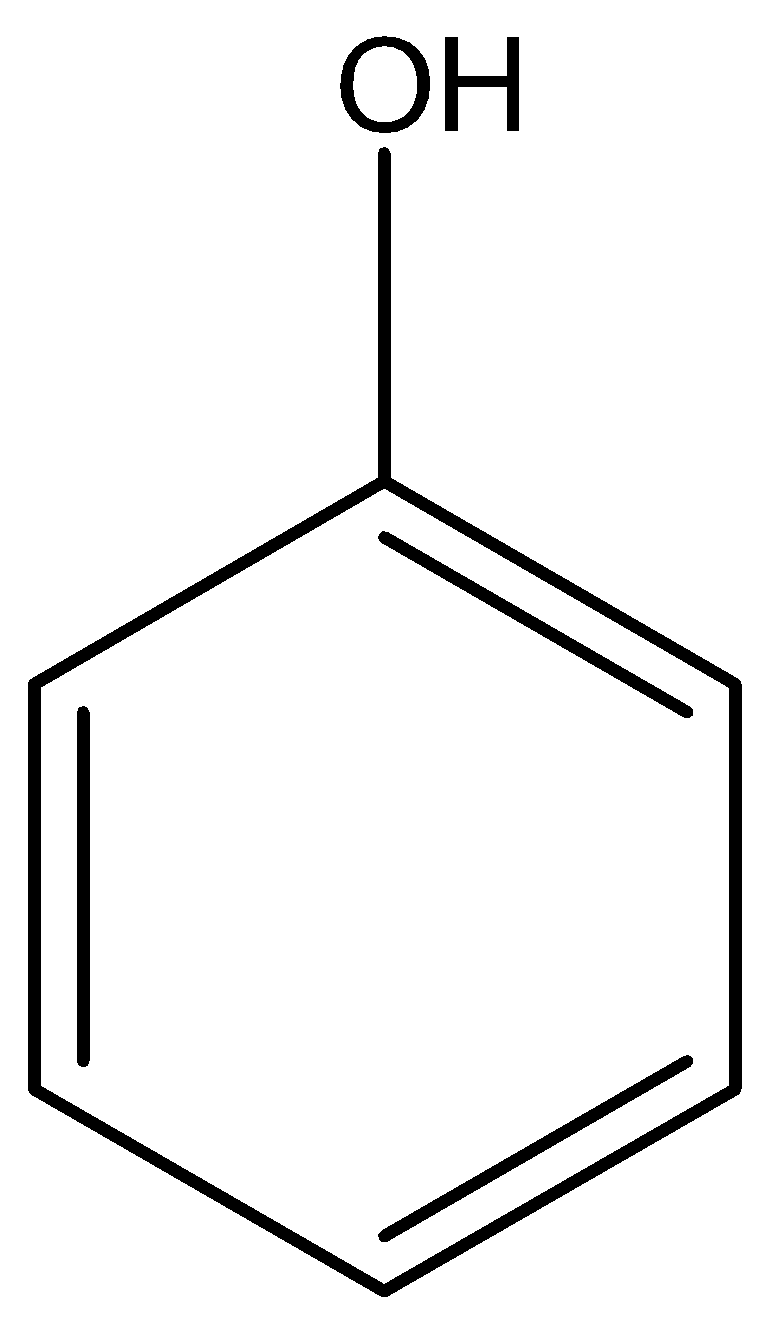
Image created in chemdraw by SME.
Phenol is a weak acid that has pH of \[8 - 12\] , there is resonance stabilization in phenol due to which it is more acidic than aliphatic alcohols.
Option A) this is an incorrect option as \[ - OH\] is a functional group of alcohol and
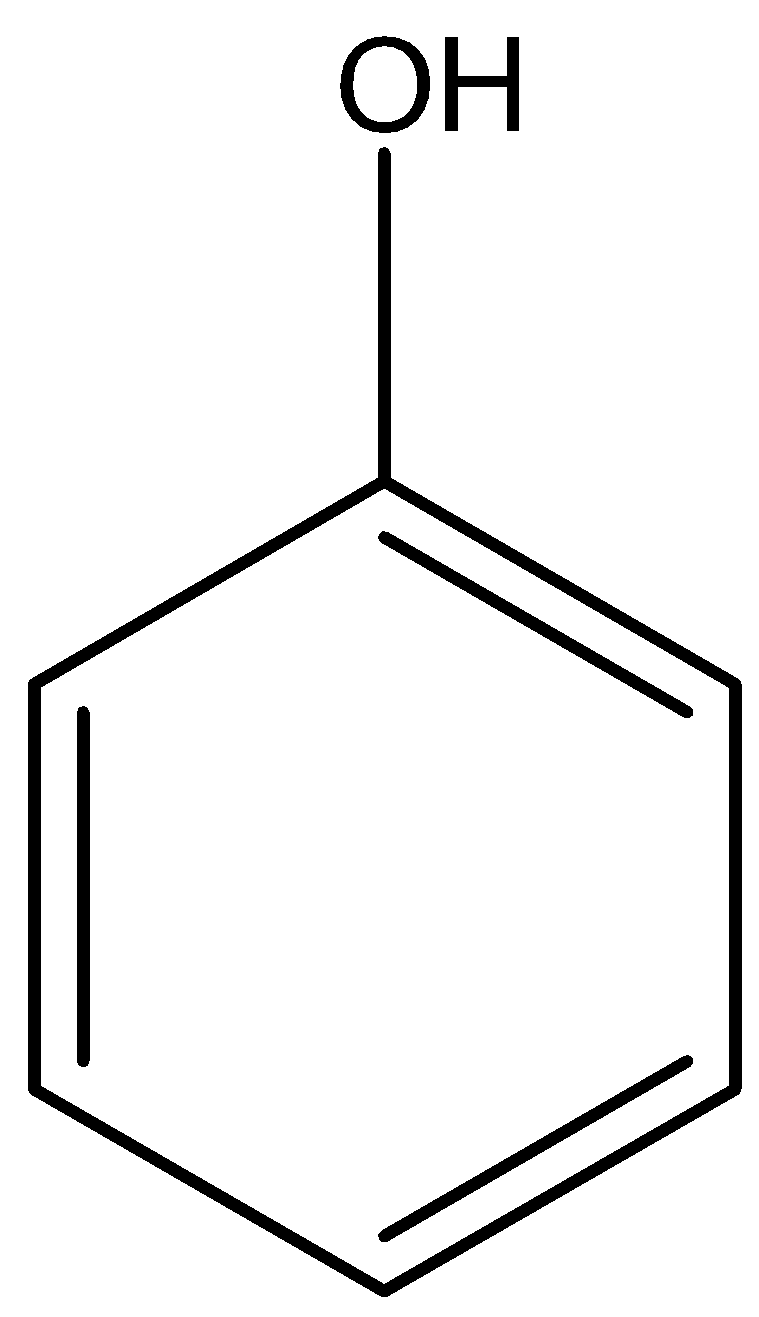 is the functional group of phenol.
is the functional group of phenol.
Option B) This is a correct option as the two represent phenol and alcohol in the correct way.
Option C) this is an incorrect option as \[ - OH\] is a functional group of alcohol but \[ - COOH\] is a functional group of carboxylic acid and not phenol.
Option D) this is an incorrect option as we get option B as the correct option.
Hence, the correct option is, ‘Option B’.
Note: We need to know that an alcohol is an aliphatic organic compound whereas Phenol is an aromatic organic compound. Phenols have generally higher acidity than alcohols.
We have to know that alcohol is an organic compound which has at least one hydroxyl group which is attached to the saturated carbon atom chain.
Complete step by step answer:
We have to remember that the nomenclature for writing the name of alcohol is Suffix “ol” is used when hydroxyl group is present as a higher priority in any compound whereas Prefix “hydroxy” is used when other groups are present as higher priority than alcohol.
Examples of alcohol:
\[{C_2}{H_5}OH\]- Ethanol
Phenol is an aromatic organic compound that contains hydroxyl group which is attached to a benzene ring therefore the functional group is \[-{C_6}{H_5}OH\]or

Image created in chemdraw by SME.
Phenol is a weak acid that has pH of \[8 - 12\] , there is resonance stabilization in phenol due to which it is more acidic than aliphatic alcohols.
Option A) this is an incorrect option as \[ - OH\] is a functional group of alcohol and

Option B) This is a correct option as the two represent phenol and alcohol in the correct way.
Option C) this is an incorrect option as \[ - OH\] is a functional group of alcohol but \[ - COOH\] is a functional group of carboxylic acid and not phenol.
Option D) this is an incorrect option as we get option B as the correct option.
Hence, the correct option is, ‘Option B’.
Note: We need to know that an alcohol is an aliphatic organic compound whereas Phenol is an aromatic organic compound. Phenols have generally higher acidity than alcohols.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




