
From the discharge tube experiment, it was concluded that:
a) Mass of proton is in fraction
b) Matter contains electrons
c) Nucleus contains positive charge
d) Positive rays are heavier than protons
Answer
592.5k+ views
Hint: The discharge tube experiment is another name for the ‘Cathode Ray Tube experiment’. The Discovery of the discharge tube revolutionized the study of atomic structure and subatomic particles.
Complete step by step answer:
Before answering this question, let us discuss the cathode ray experiment.
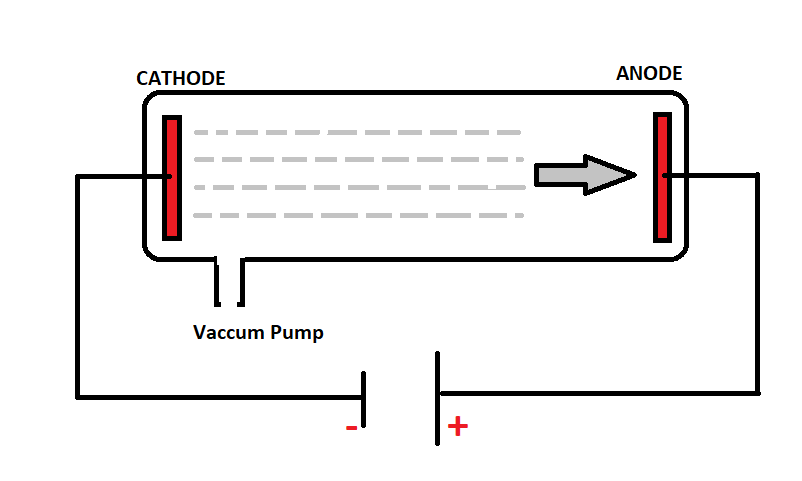
Discharge tube is also known as ‘CROOKES TUBE’. This tube is made up of a glass which has two metallic plates. One end is connected to the positive terminal and the other to the negative terminal of the high voltage power supply. Plate connected to the positive terminal is anode and the plate connected to the negative terminal is the cathode and the tube is filled with any gas.
In the discharge tube experiment, at a very high voltage and low pressure, an electrical current was passed. Due to this electrical current, a stream of rays originating from the cathode passed through the tube. These rays were called the cathode rays.
Experiments on the discharge tube were first performed in 1878 by an English physicist, William Crookes. Later in the 19th century, J. J. Thomson studied the characteristics and the constituents of cathode rays.
Certain properties of these cathode rays were concluded so let us discuss them now.
- Cathode rays originated from the cathode and travelled in straight lines carrying a negative charge.
- The cathode rays were deflected by electrical and magnetic fields. This behaviour was similar to that of a negatively charged particle and this suggested that the rays contained electrons.
- These rays consisted of material particles, electrons and it caused other materials to glow upon striking on them.
Through this experiment it was concluded that electrons were the basic constituent of the atoms and matter contains atoms thus it was concluded that matter consisted of electrons.
We can understand from the above discussion that it was concluded about every atom and thus matter containing electrons.
So, the correct answer is “Option B”.
Note: The Crookes tube led to the discovery of X-rays which has many applications and practical uses. When a voltage of around 5000V or greater was applied, it accelerated the electrons and thus they had high velocity and upon hitting the anode or the glass wall of the tube, X-ray was generated.
Complete step by step answer:
Before answering this question, let us discuss the cathode ray experiment.

Discharge tube is also known as ‘CROOKES TUBE’. This tube is made up of a glass which has two metallic plates. One end is connected to the positive terminal and the other to the negative terminal of the high voltage power supply. Plate connected to the positive terminal is anode and the plate connected to the negative terminal is the cathode and the tube is filled with any gas.
In the discharge tube experiment, at a very high voltage and low pressure, an electrical current was passed. Due to this electrical current, a stream of rays originating from the cathode passed through the tube. These rays were called the cathode rays.
Experiments on the discharge tube were first performed in 1878 by an English physicist, William Crookes. Later in the 19th century, J. J. Thomson studied the characteristics and the constituents of cathode rays.
Certain properties of these cathode rays were concluded so let us discuss them now.
- Cathode rays originated from the cathode and travelled in straight lines carrying a negative charge.
- The cathode rays were deflected by electrical and magnetic fields. This behaviour was similar to that of a negatively charged particle and this suggested that the rays contained electrons.
- These rays consisted of material particles, electrons and it caused other materials to glow upon striking on them.
Through this experiment it was concluded that electrons were the basic constituent of the atoms and matter contains atoms thus it was concluded that matter consisted of electrons.
We can understand from the above discussion that it was concluded about every atom and thus matter containing electrons.
So, the correct answer is “Option B”.
Note: The Crookes tube led to the discovery of X-rays which has many applications and practical uses. When a voltage of around 5000V or greater was applied, it accelerated the electrons and thus they had high velocity and upon hitting the anode or the glass wall of the tube, X-ray was generated.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




