
From the above mentioned periodic table (abbreviated), the element with the smallest ionic radius is:
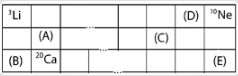
(A) A
(B) B
(C) C
(D) D
(E) E

Answer
587.4k+ views
Hint:Ions are the charged species : there are two type of ions ie. Cation and anion. Ionic radius is measured as the distance between cations and anions in an ionic crystal. Ionic radii show different trends along the period and down the group.
Complete step by step answer:
The trends shown by ionic radius are :-
1. Down the group :-
When we move down the group, new shells are added in the atoms and the number of electrons increases. Due to addition of shells, the ionic radius of atoms increases.
2. Across a period :-
As we move from left to right in a group, the ionic radius first decreases then increases and then again decreases. For metals the ionic radius decreases as cations are formed by them. Metals lose their electron (S) to form cation due to which, nuclear change per electron increases and electrons get tightly held within the atom. That is why it is said that cations are smaller than their parent atom.
For non-metals, ionic radius increases as they form anions nonmetals gain electron (S) to form anions due to which the nuclear charge per electron decreases and electrons become less tightly held within the atom. Therefore, it is said that anions are larger in size as compared to its parent atom.
In the given question, the elements A and C are present below the D. and in turn B and E are present below A and C. Due to the position down in the group, their ionic radius goes on increasing.
Whereas, if we see across the period the atoms are arranged as B, then A, then C, then D and E at last.
E is a noble gas as it falls in the group of neon (a noble gas).
Therefore, we can say that the element D has the smallest ionic radius because it is present in the top period and second last group.
Hence, the correct option is (D) .
Note:
In anions, the ionic radii is larger than its parent atom. This is because the net repulsion of electrons will outweigh the nuclear charge and the ion will become large in size.
Complete step by step answer:
The trends shown by ionic radius are :-
1. Down the group :-
When we move down the group, new shells are added in the atoms and the number of electrons increases. Due to addition of shells, the ionic radius of atoms increases.
2. Across a period :-
As we move from left to right in a group, the ionic radius first decreases then increases and then again decreases. For metals the ionic radius decreases as cations are formed by them. Metals lose their electron (S) to form cation due to which, nuclear change per electron increases and electrons get tightly held within the atom. That is why it is said that cations are smaller than their parent atom.
For non-metals, ionic radius increases as they form anions nonmetals gain electron (S) to form anions due to which the nuclear charge per electron decreases and electrons become less tightly held within the atom. Therefore, it is said that anions are larger in size as compared to its parent atom.
In the given question, the elements A and C are present below the D. and in turn B and E are present below A and C. Due to the position down in the group, their ionic radius goes on increasing.
Whereas, if we see across the period the atoms are arranged as B, then A, then C, then D and E at last.
E is a noble gas as it falls in the group of neon (a noble gas).
Therefore, we can say that the element D has the smallest ionic radius because it is present in the top period and second last group.
Hence, the correct option is (D) .
Note:
In anions, the ionic radii is larger than its parent atom. This is because the net repulsion of electrons will outweigh the nuclear charge and the ion will become large in size.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




