
Formula of Freon is:
A. ${\text{CC}}{{\text{l}}_{\text{2}}}{{\text{F}}_{\text{2}}}$
B. ${\text{COC}}{{\text{l}}_{\text{2}}}$
C. ${\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}$
D. None of these
Answer
603.6k+ views
Hint: For knowing the formula of Freon we need to know the chemical name of the compound. Knowing the structure will also be an added advantage.
Step by step answer:
The chemical name of Freon is Dichlorofluoromethane. The structure of Freon is given below:
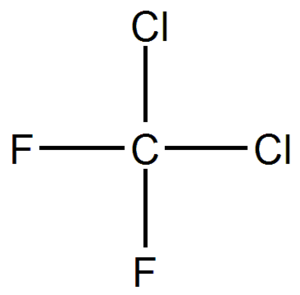
As we can see from the diagram, there are two fluorine atoms, two chlorine atoms and one carbon atom. Therefore, the formula of the compound will be ${\text{CC}}{{\text{l}}_{\text{2}}}{{\text{F}}_{\text{2}}}$.
As we can see, the formula resembles one of the options of the questions, which is option A. So the correct option is Option A.
Additional Information: Here are some properties of Freon, that we should be knowing:
Freon is slightly soluble in water at a level of approximately 0.3 grams per litre at room temperature.
Freon is tasteless, odourless (in most cases).
Because Freon is non-toxic in nature, it eliminates the danger posed by refrigerator leaks and to find the replacement for the dangerous refrigerants then in use.
Inhalation of Freon is toxic because inhalation of Freon may cause the cut down of vital oxygen to the cells and the lungs.
It has a very low melting point of $- 158 ^{\circ}C$ and boiling point of $-30^{\circ}C$.
Note: It is always advised that we should be able to know the structure of the compound, before naming it or writing the formula. Even knowing the name, would be enough, as because we can easily derive the structure following the nomenclature rules.
Step by step answer:
The chemical name of Freon is Dichlorofluoromethane. The structure of Freon is given below:

As we can see from the diagram, there are two fluorine atoms, two chlorine atoms and one carbon atom. Therefore, the formula of the compound will be ${\text{CC}}{{\text{l}}_{\text{2}}}{{\text{F}}_{\text{2}}}$.
As we can see, the formula resembles one of the options of the questions, which is option A. So the correct option is Option A.
Additional Information: Here are some properties of Freon, that we should be knowing:
Freon is slightly soluble in water at a level of approximately 0.3 grams per litre at room temperature.
Freon is tasteless, odourless (in most cases).
Because Freon is non-toxic in nature, it eliminates the danger posed by refrigerator leaks and to find the replacement for the dangerous refrigerants then in use.
Inhalation of Freon is toxic because inhalation of Freon may cause the cut down of vital oxygen to the cells and the lungs.
It has a very low melting point of $- 158 ^{\circ}C$ and boiling point of $-30^{\circ}C$.
Note: It is always advised that we should be able to know the structure of the compound, before naming it or writing the formula. Even knowing the name, would be enough, as because we can easily derive the structure following the nomenclature rules.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




