
Formaldehyde reacts with ammonia to give:
A. $C{{H}_{2}}=NH$
B.
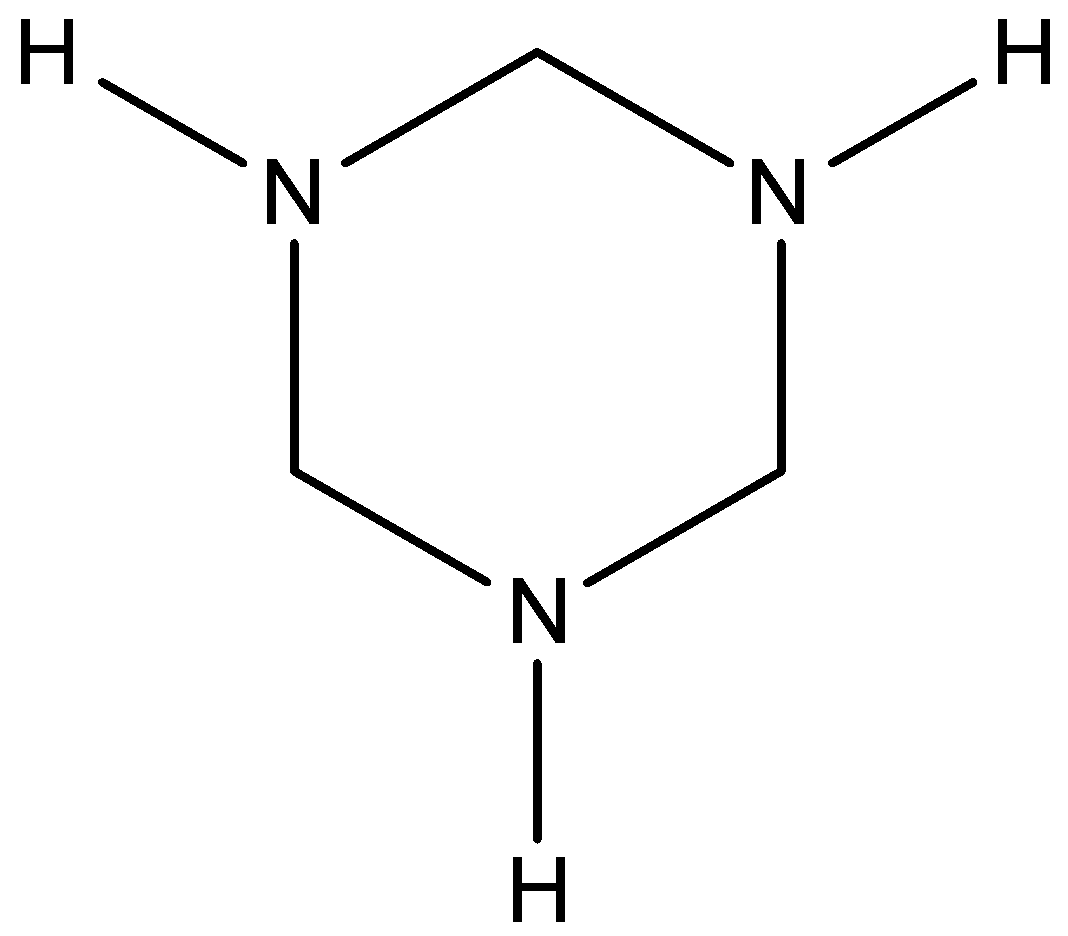
C.
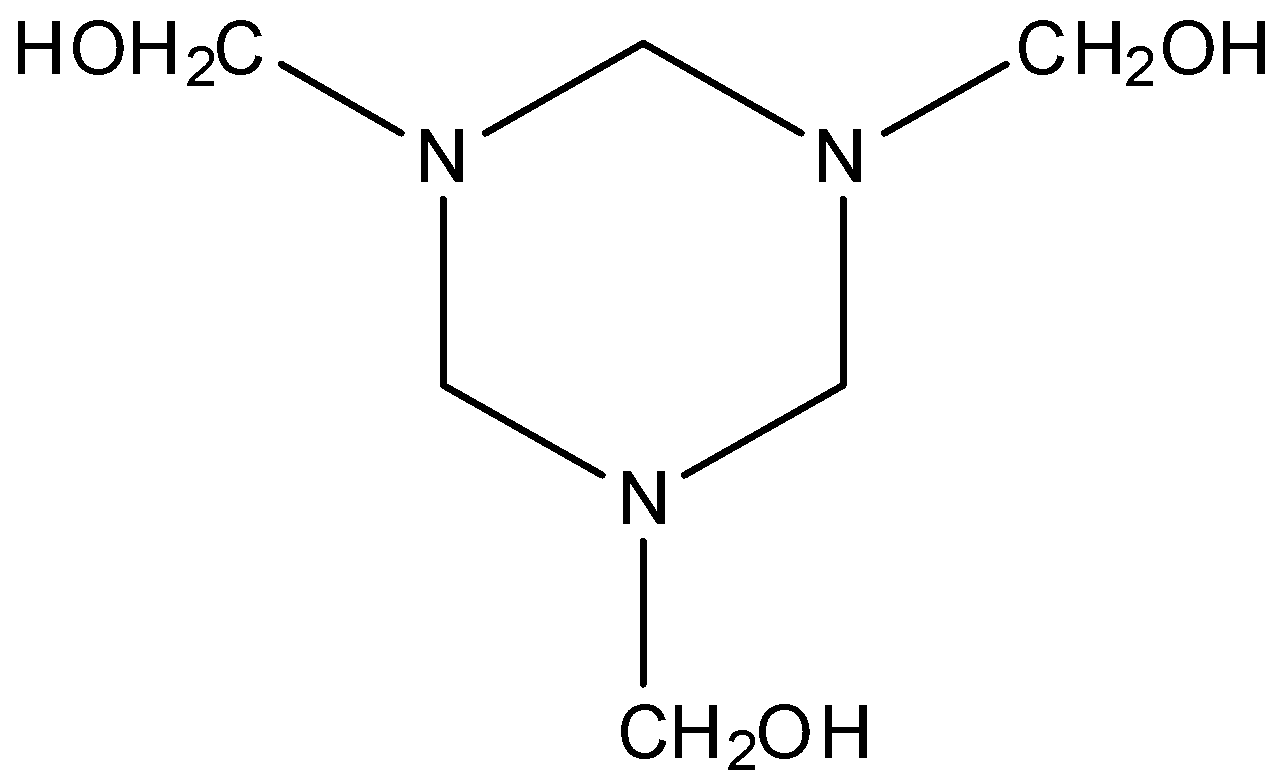
D. Hexamethylenetetramine


Answer
578.4k+ views
Hint: Aliphatic aldehydes react with ammonia and forms aldehyde ammonias. Formaldehyde an aliphatic aldehyde reacts with ammonia in slightly alkaline or neutral medium and forms amino methanol as the initial product. Later amino methanol undergoes reaction with formaldehyde and forms a methylene derivative as the product.
Complete answer:
- In the question it is asked to find the product when formaldehyde reacts with ammonia.
- The molecular formula of formaldehyde is HCHO and the molecular formula of ammonia is $N{{H}_{3}}$ .
- The reaction of formaldehyde with ammonia is as follows.
\[6HCHO+4N{{H}_{3}}\to \underset{Hexamethylenetetra\min e}{\mathop{{{(C{{H}_{2}})}_{6}}{{N}_{4}}}}\,+6{{H}_{2}}O\]
- We can see that six moles of formaldehyde reacts with four moles of ammonia and forms one mole of Hexamethylenetetramine and six moles of water as the products.
- The structure of Hexamethylenetetramine is as follows.
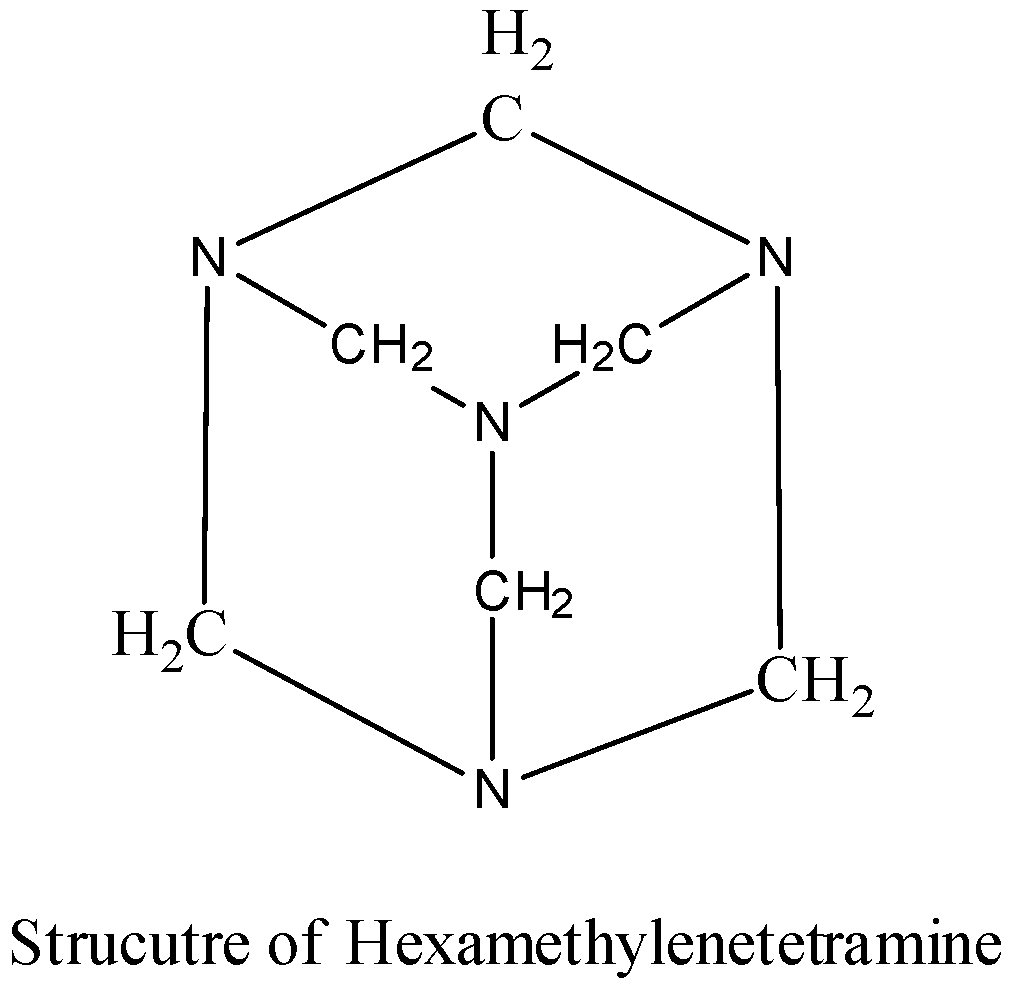
- Therefore the product formed when formaldehyde reacts with ammonia is hexamethylenetetramine.
So, the correct option is D.
Additional information:
- Hexamethylenetetramine is a heterocyclic compound.
- Hexamethylenetetramine also called hexamine or urotropin.
- Hexamethylenetetramine is a crystalline compound and soluble in water and in several organic polar solvents like carbon tetrachloride and etc.
- Hexamethylenetetramine has a cage-like structure.
- Hexamethylenetetramine is used to treat urinary tract infections.
Note:
Reaction of formaldehyde with ammonia is an example of condensation reaction. During the reaction of formaldehyde with ammonia there is a release of water molecules as the byproducts. In condensation reaction only byproducts are going to form as the side products.
Complete answer:
- In the question it is asked to find the product when formaldehyde reacts with ammonia.
- The molecular formula of formaldehyde is HCHO and the molecular formula of ammonia is $N{{H}_{3}}$ .
- The reaction of formaldehyde with ammonia is as follows.
\[6HCHO+4N{{H}_{3}}\to \underset{Hexamethylenetetra\min e}{\mathop{{{(C{{H}_{2}})}_{6}}{{N}_{4}}}}\,+6{{H}_{2}}O\]
- We can see that six moles of formaldehyde reacts with four moles of ammonia and forms one mole of Hexamethylenetetramine and six moles of water as the products.
- The structure of Hexamethylenetetramine is as follows.

- Therefore the product formed when formaldehyde reacts with ammonia is hexamethylenetetramine.
So, the correct option is D.
Additional information:
- Hexamethylenetetramine is a heterocyclic compound.
- Hexamethylenetetramine also called hexamine or urotropin.
- Hexamethylenetetramine is a crystalline compound and soluble in water and in several organic polar solvents like carbon tetrachloride and etc.
- Hexamethylenetetramine has a cage-like structure.
- Hexamethylenetetramine is used to treat urinary tract infections.
Note:
Reaction of formaldehyde with ammonia is an example of condensation reaction. During the reaction of formaldehyde with ammonia there is a release of water molecules as the byproducts. In condensation reaction only byproducts are going to form as the side products.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




