
When formaldehyde is treated with ethylamine, it gives:
A. ethyl alcohol
B. propionaldehyde
C. dimethylamine
D. ethyl methyl imine
Answer
521.7k+ views
Hint: Formaldehyde is the simplest aldehyde, which is the organic compound with (CHO) functional group. Ethyl amines are the compounds having the functional group amine$\left( -N{{H}_{2}} \right)$. The reaction between aldehyde and amine is a type of reversible reaction that results in the formation of imines having the $CH=NH$ group.
Complete answer:
The reaction between formaldehyde, the simplest aldehyde and the amine functional group that is a derivative of ammonia is carried by a reversible process. Formaldehyde and ethylamine react together in the presence of acid to form a compound called substituted imine that has the $CH=NH$ group. This imine is also known as a Schiff base.
The reaction between formaldehyde and ethyl amine is as follows:
$HCHO+{{C}_{2}}{{H}_{5}}N{{H}_{2}}{{H}_{2}}C=N{{C}_{2}}{{H}_{5}}+{{H}_{2}}O$
The compound formed is known as ethyl methyl imine. The mechanism of the reaction involves a reversible reaction, but due to the formation of water, it will shift in the forward direction. The mechanism is:
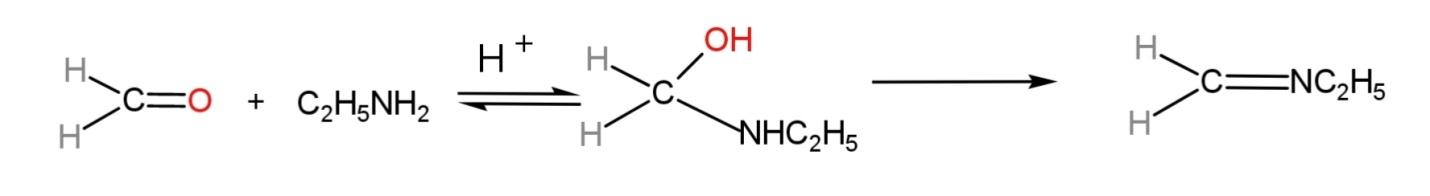
Hence, formaldehyde treated with ethyl amine gives ethyl methyl imine.
So option D is correct.
Note:
The process is reversible because ammonia and its derivatives can be decomposed in acid or alkali giving back pure aldehyde. The imine formed here is a Schiff base that is used in various organic syntheses and also as intermediate or catalysts. Schiff bases also act as important ligands in coordination compounds. Ketones also react in the same way as aldehyde to form keto imines.
Complete answer:
The reaction between formaldehyde, the simplest aldehyde and the amine functional group that is a derivative of ammonia is carried by a reversible process. Formaldehyde and ethylamine react together in the presence of acid to form a compound called substituted imine that has the $CH=NH$ group. This imine is also known as a Schiff base.
The reaction between formaldehyde and ethyl amine is as follows:
$HCHO+{{C}_{2}}{{H}_{5}}N{{H}_{2}}{{H}_{2}}C=N{{C}_{2}}{{H}_{5}}+{{H}_{2}}O$
The compound formed is known as ethyl methyl imine. The mechanism of the reaction involves a reversible reaction, but due to the formation of water, it will shift in the forward direction. The mechanism is:

Hence, formaldehyde treated with ethyl amine gives ethyl methyl imine.
So option D is correct.
Note:
The process is reversible because ammonia and its derivatives can be decomposed in acid or alkali giving back pure aldehyde. The imine formed here is a Schiff base that is used in various organic syntheses and also as intermediate or catalysts. Schiff bases also act as important ligands in coordination compounds. Ketones also react in the same way as aldehyde to form keto imines.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




