
: For which one of the following neutralizations, would the indicator methyl red be useful?
A.${\text{NaOH + C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$
B.${\text{HCl + N}}{{\text{H}}_3}$
C.${\text{HCl + NaOH}}$
D. None of the above
Answer
591.6k+ views
Hint: Methyl red is an acid-base indicator which is used for titration of strong acid with a weak base. It changes colour when the pH of the solution changes.
Complete step by step answer:
Methyl red is an anionic azo dye which is used for titration of strong acid with a weak base.
In option A, sodium hydroxide is a strong base and acetic acid is a weak base So methyl red cannot be used in this neutralization reaction.
In option B, hydrochloric acid is a strong acid but ammonia is a weak base. So methyl red can be used as an indicator in this neutralization. The reaction is as follows-
${\text{HCl + N}}{{\text{H}}_{\text{3}}} \to {\text{N}}{{\text{H}}_4}{\text{Cl}}$
Ammonium chloride is formed in this reaction which is a salt of strong acid and weak base. Its pH will be between $4.6$ to $6.0$ which will be suitable for using methyl red.
In option C, sodium hydroxide is a strong base and hydrochloric acid is a strong acid so methyl red cannot be used in this neutralization reaction.
Hence the correct answer is B.
Additional Information:
Methyl Red is an azo dye with chemical formula ${{\text{C}}_{15}}{{\text{H}}_{15}}{{\text{N}}_3}{{\text{O}}_2}$.Its structure is-
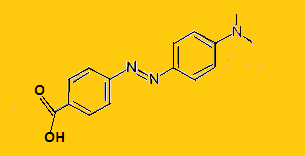
It is a yellow weak acid. It gives red color in acidic medium and yellow in basic medium. It is insoluble in water and soluble in organic solvents. It is obtained by diazotization of anthranilic acid and dimethyl-aniline.
Note:
It is for following purposes-
-It is used as a colouring agent in paints, inks.
-It is also used as an indicator and reagent.
-It is used for titrating ammonia, weak organic bases, and only suitable for oxalic acid and picric acid.
Complete step by step answer:
Methyl red is an anionic azo dye which is used for titration of strong acid with a weak base.
In option A, sodium hydroxide is a strong base and acetic acid is a weak base So methyl red cannot be used in this neutralization reaction.
In option B, hydrochloric acid is a strong acid but ammonia is a weak base. So methyl red can be used as an indicator in this neutralization. The reaction is as follows-
${\text{HCl + N}}{{\text{H}}_{\text{3}}} \to {\text{N}}{{\text{H}}_4}{\text{Cl}}$
Ammonium chloride is formed in this reaction which is a salt of strong acid and weak base. Its pH will be between $4.6$ to $6.0$ which will be suitable for using methyl red.
In option C, sodium hydroxide is a strong base and hydrochloric acid is a strong acid so methyl red cannot be used in this neutralization reaction.
Hence the correct answer is B.
Additional Information:
Methyl Red is an azo dye with chemical formula ${{\text{C}}_{15}}{{\text{H}}_{15}}{{\text{N}}_3}{{\text{O}}_2}$.Its structure is-

It is a yellow weak acid. It gives red color in acidic medium and yellow in basic medium. It is insoluble in water and soluble in organic solvents. It is obtained by diazotization of anthranilic acid and dimethyl-aniline.
Note:
It is for following purposes-
-It is used as a colouring agent in paints, inks.
-It is also used as an indicator and reagent.
-It is used for titrating ammonia, weak organic bases, and only suitable for oxalic acid and picric acid.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




