
For the preparation of chloroethane
A. HCl gas is passed through ethanol.
B. ethanol is treated with thionyl chloride in the presence of dimethyl amine or pyridine.
C. Ethyl sulphide is treated with hydrogen chloride.
D. Any of the above methods can be employed.
Answer
577.8k+ views
Hint:To prepare chloroethane from ethanol, reduction of alcohol group is required. Ethanol gives nucleophilic substitution with hydrochloric acid. But this reaction requires a catalyst. Thionyl forms ethoxide ion and dimethyl amine or pyridine helps to generate a chloride nucleophile.
Complete answer:
Alcohol gives nucleophilic substitution with hydrochloric acid. But this reaction requires a catalyst such as zinc chloride. The tertiary alcohol is more reactive for this reaction than the primary alcohol. Ethanol is the primary alcohol and the catalyst is also not present, so chloroethane cannot be prepared by passing the hydrochloric gas through ethanol.
Reaction, when ethanol is treated with thionyl chloride in the presence of dimethyl amine or pyridine, is as follows:
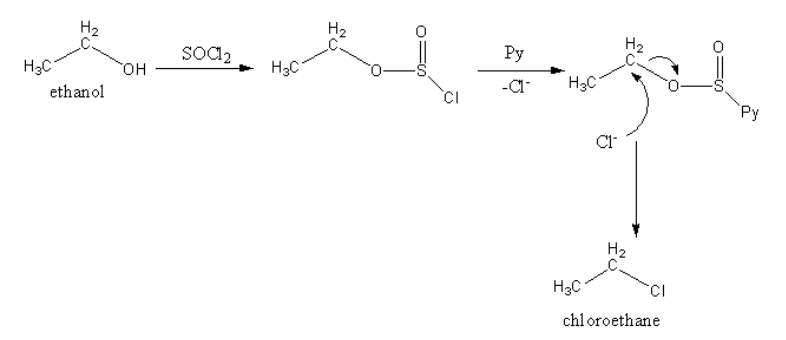
Ethyl sulphide on treating with hydrogen chloride does not give chloroethane.
So, for the preparation of chloroethane, ethanol is treated with thionyl chloride in the presence of dimethyl amine or pyridine.
Therefore, option (B) ethanol is treated with thionyl chloride in the presence of dimethyl amine or pyridine, is correct.
Note:
The reaction of an alcohol with thionyl chloride to generate alkyl halide is known as the Darzens process. The reaction of an alcohol with hydrochloric acid in presence of a catalyst such as zinc chloride is known as the Lucas test. This test is used to differentiate alcohols. This is a nucleophilic substitution reaction, so a carbocation generates during the reaction so, the rate of reaction depends upon the stability of the carbocation. Thus Lucas tests differentiate primary, secondary and tertiary alcohols.
Complete answer:
Alcohol gives nucleophilic substitution with hydrochloric acid. But this reaction requires a catalyst such as zinc chloride. The tertiary alcohol is more reactive for this reaction than the primary alcohol. Ethanol is the primary alcohol and the catalyst is also not present, so chloroethane cannot be prepared by passing the hydrochloric gas through ethanol.
Reaction, when ethanol is treated with thionyl chloride in the presence of dimethyl amine or pyridine, is as follows:

Ethyl sulphide on treating with hydrogen chloride does not give chloroethane.
So, for the preparation of chloroethane, ethanol is treated with thionyl chloride in the presence of dimethyl amine or pyridine.
Therefore, option (B) ethanol is treated with thionyl chloride in the presence of dimethyl amine or pyridine, is correct.
Note:
The reaction of an alcohol with thionyl chloride to generate alkyl halide is known as the Darzens process. The reaction of an alcohol with hydrochloric acid in presence of a catalyst such as zinc chloride is known as the Lucas test. This test is used to differentiate alcohols. This is a nucleophilic substitution reaction, so a carbocation generates during the reaction so, the rate of reaction depends upon the stability of the carbocation. Thus Lucas tests differentiate primary, secondary and tertiary alcohols.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




