
For the following reaction product is:
${{\text{H}}_{\text{3}}}{\text{C}} - {\text{CH}}\,{\text{ =
}}\,{\text{C}}{{\text{H}}_{\text{2}}}\, + \,{\text{HCl}} \to $
Answer
571.8k+ views
Hint: Hydrogen halide gives an addition reaction to alkene. Alkene behaves as nucleophilic so, gets protonated by hydrogen halide. Then at carbocation halide attacks. So, alkyl halide forms as a product. The formation of the product is decided based on the Markovnikov rule.
Complete step by step solution:
The reaction of hydrogen chloride with alkene is an example of an electrophilic addition reaction. In the first step, the electrophilic addition takes place. In the second step, the nucleophilic addition takes place.
Hydrogen chloride gives an addition reaction on propene. During the reaction, first, alkene attacks on the hydrogen of hydrogen chloride, so a carbocation forms. On this carbocation, chloride gets attached.
According to the Markovnikov rule, the negative part of the attacking group gets attached to the position where less number of hydrogens are present.
The product formation is shown as follows:
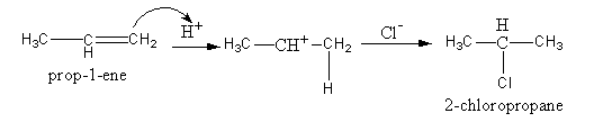
So, by the reaction of propene with hydrogen chloride, the product formed is $2 - $ chloropropane. In product, the negative chloride ion is attached to carbon that has only one hydrogen atom.
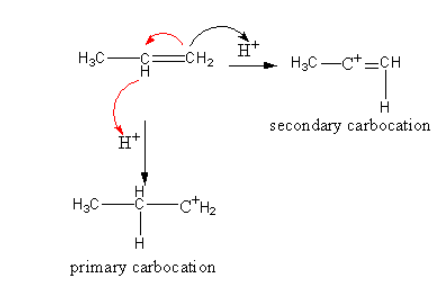
Note: Markovnikov rule is based upon the observation of stability of the carbocation which formed during the reaction. In the case of unsymmetrical alkene two possibilities are there for the formation of a carbocation.
Which are shown as follows:
In secondary carbocation, the more electron-donating groups are present which stabilized the positive charge of the carbocation. So secondary carbocation is more stable than primary, so the reaction goes through the formation of a secondary carbocation.
The stability order of carbocation is as follows:
Tertiary carbocation > secondary carbocation > primary carbocation.
Marcovnikov's rule can also be stated as that hydrogen of the attacking group gets attached where hydrogens are present in more numbers.
Complete step by step solution:
The reaction of hydrogen chloride with alkene is an example of an electrophilic addition reaction. In the first step, the electrophilic addition takes place. In the second step, the nucleophilic addition takes place.
Hydrogen chloride gives an addition reaction on propene. During the reaction, first, alkene attacks on the hydrogen of hydrogen chloride, so a carbocation forms. On this carbocation, chloride gets attached.
According to the Markovnikov rule, the negative part of the attacking group gets attached to the position where less number of hydrogens are present.
The product formation is shown as follows:

So, by the reaction of propene with hydrogen chloride, the product formed is $2 - $ chloropropane. In product, the negative chloride ion is attached to carbon that has only one hydrogen atom.
Note: Markovnikov rule is based upon the observation of stability of the carbocation which formed during the reaction. In the case of unsymmetrical alkene two possibilities are there for the formation of a carbocation.
Which are shown as follows:
In secondary carbocation, the more electron-donating groups are present which stabilized the positive charge of the carbocation. So secondary carbocation is more stable than primary, so the reaction goes through the formation of a secondary carbocation.
The stability order of carbocation is as follows:

Tertiary carbocation > secondary carbocation > primary carbocation.
Marcovnikov's rule can also be stated as that hydrogen of the attacking group gets attached where hydrogens are present in more numbers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




