
For the conversion of aniline to N-methyl aniline, the reagent used is:
A.${\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl/Anhyd}}{\text{.AlC}}{{\text{l}}_3}$
B.${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{Cl}}$
C.${\text{C}}{{\text{H}}_{\text{4}}}$
D.${\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}$
Answer
588k+ views
Hint: N - methyl aniline is an alkylated aniline derivative. An alkyl group can be introduced into any molecule by the process of alkylation by allowing the molecule to react with alkyl halides.
Complete step by step answer:
Due to the presence of a lone pair of electrons on the nitrogen atom, amines are very good nucleophiles and hence have the ability to react with a variety of electrophiles (which are electron deficient compounds). The electrophiles may be metal ions or alkyl halides or acid chlorides.
When the amines react with alkyl halides as electrophiles, an alkyl group is inserted into the amine molecules and the process is termed as alkylation. For example, all the three classes of aliphatic amines undergo alkylation reactions via nucleophilic substitution reactions on alkyl halides. Hence, a primary aliphatic amine can be converted into secondary and tertiary aliphatic amine and ultimately into quaternary ammonium salts. The alkylation of ethylamine with ethyl bromide is shown below as an example.
$\mathop {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}}\limits_{{\text{Ethylamine}}} {\text{ + }}\mathop {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}}\limits_{{\text{Ethyl Bromide}}} \to \mathop {{{\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}} \right)}_2}{\text{NH}}}\limits_{{\text{Diethylamine}}} {\text{ + HBr}}$
Now, aniline is an aromatic amine and has the structure shown below.
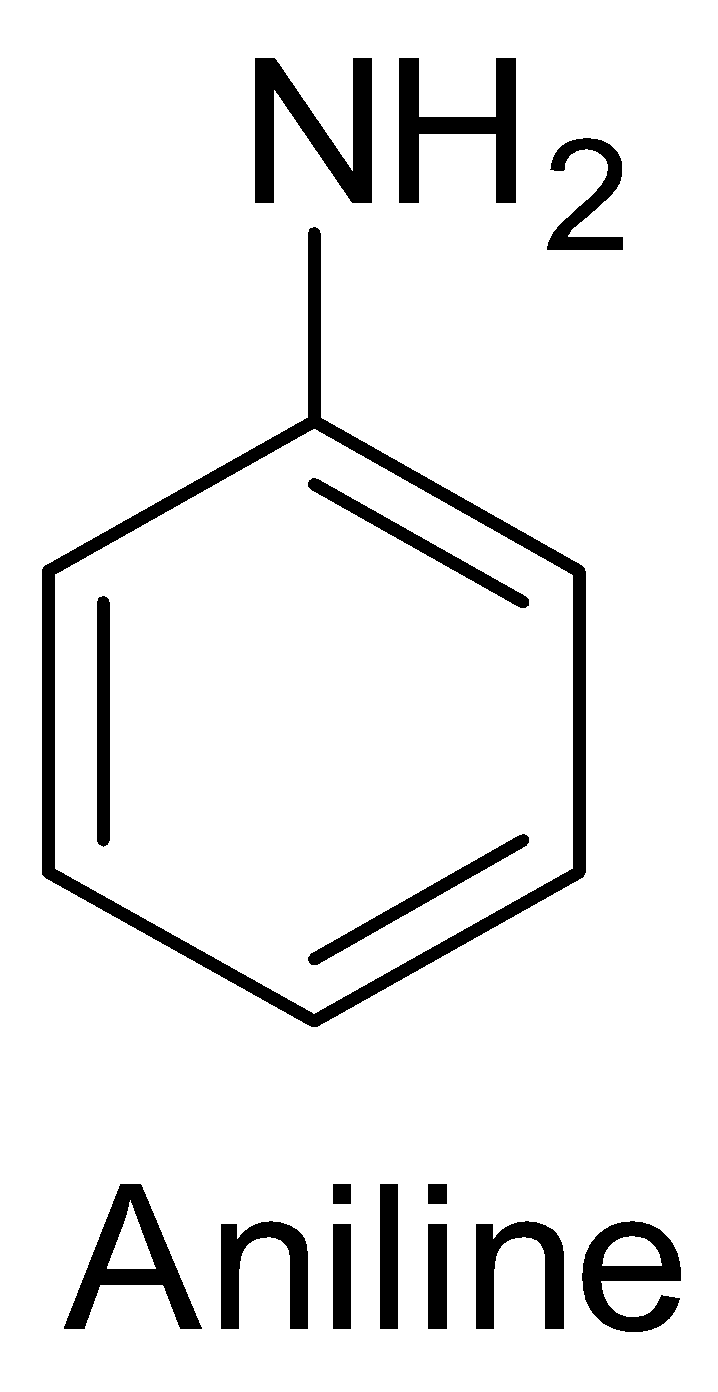
N – methyl aniline has the following structure.

Thus, it is an alkylated aniline.
Just like aliphatic amines, aromatic amines will also undergo alkylation when treated with alkyl halides. When aniline is treated with methyl chloride, one hydrogen atom attached to the nitrogen atom of aniline will be replaced by the methyl group of methyl chloride. The product formed will be N – methyl aniline.
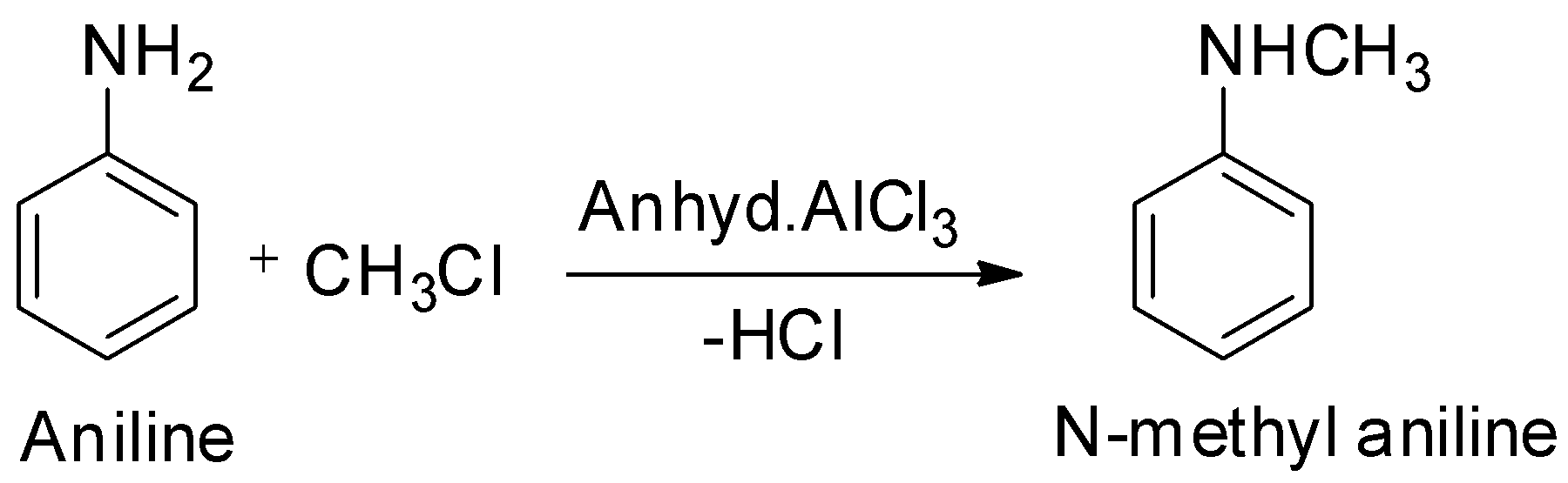
Hence, option A is correct.
Note:
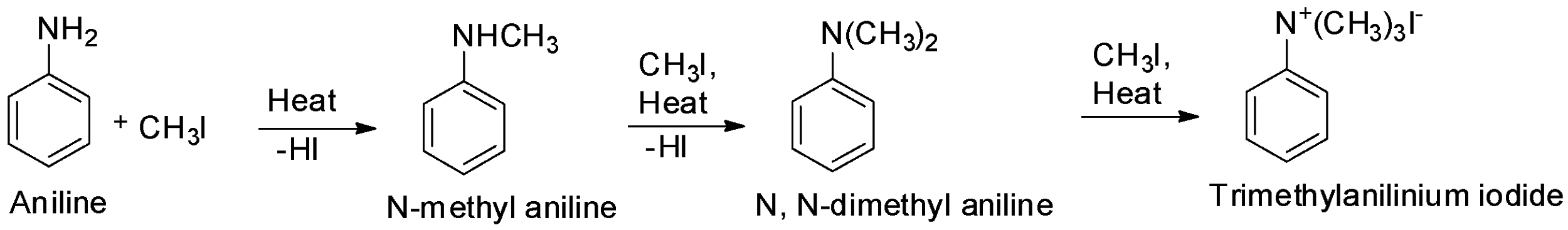
In presence of excess alkyl halide, the N-methyl aniline can be further converted into N, N- dimethyl aniline and trimethylanilinium halide. The process of converting an amine (primary, secondary or tertiary) into its quaternary ammonium salt on treatment with excess of an alkyl halide is called exhaustive alkylation. If methyl iodide is the alkyl halide used, then the process is most commonly called ‘exhaustive methylation’.
Complete step by step answer:
Due to the presence of a lone pair of electrons on the nitrogen atom, amines are very good nucleophiles and hence have the ability to react with a variety of electrophiles (which are electron deficient compounds). The electrophiles may be metal ions or alkyl halides or acid chlorides.
When the amines react with alkyl halides as electrophiles, an alkyl group is inserted into the amine molecules and the process is termed as alkylation. For example, all the three classes of aliphatic amines undergo alkylation reactions via nucleophilic substitution reactions on alkyl halides. Hence, a primary aliphatic amine can be converted into secondary and tertiary aliphatic amine and ultimately into quaternary ammonium salts. The alkylation of ethylamine with ethyl bromide is shown below as an example.
$\mathop {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}}\limits_{{\text{Ethylamine}}} {\text{ + }}\mathop {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}}\limits_{{\text{Ethyl Bromide}}} \to \mathop {{{\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}} \right)}_2}{\text{NH}}}\limits_{{\text{Diethylamine}}} {\text{ + HBr}}$
Now, aniline is an aromatic amine and has the structure shown below.

N – methyl aniline has the following structure.

Thus, it is an alkylated aniline.
Just like aliphatic amines, aromatic amines will also undergo alkylation when treated with alkyl halides. When aniline is treated with methyl chloride, one hydrogen atom attached to the nitrogen atom of aniline will be replaced by the methyl group of methyl chloride. The product formed will be N – methyl aniline.

Hence, option A is correct.
Note:
In presence of excess alkyl halide, the N-methyl aniline can be further converted into N, N- dimethyl aniline and trimethylanilinium halide. The process of converting an amine (primary, secondary or tertiary) into its quaternary ammonium salt on treatment with excess of an alkyl halide is called exhaustive alkylation. If methyl iodide is the alkyl halide used, then the process is most commonly called ‘exhaustive methylation’.

Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




