
For the acid indicator thymol blue, pH is 2.0 when half the indicator is in unionised form. Find the % of indicator in unionised form in the solution with $[{{H}^{+}}]\text{ = 4 x 1}{{\text{0}}^{-3}}\text{ M}$.
(A) 25.4%
(B) 27.2%
(C) 28.6%
(D) 29.4%
Answer
593.7k+ views
Hint: Write the dissociation reaction for the acid indicator, thymol blue. Since the indicator is only half ionized, concentration of products and reactants will be numerically equal. We can then substitute these values in the formula mentioned below:
$\text{pH = p}{{\text{K}}_{In}}\text{ + log}\frac{[(\text{conjugate ion) }\!\!]\!\!\text{ }}{[(\text{acid indicator) }\!\!]\!\!\text{ }}$
Where,
pH is potenz of hydrogen of the solution
$\text{p}{{\text{K}}_{In}}$ is dissociation constant for acid indicator
Complete step-by-step answer:
Thymol blue is a brownish-green or reddish-brown powder in crystalline form. The formula name for thymol blue is thymol sulfonephthalein. It is commonly used as a pH indicator.
Thymol blue is insoluble in water. However, it is readily soluble when alcohol is used as the solvent or very dilute alkali solutions as well.
Thymol blue changes its color from red to yellow between the range of pH 1.2 - 2.8. Then the color changes from yellow to blue between 8.0 - 9.6 pH values. It is an important component of universal indicators.
We are now going to calculate the % of indicator in unionised form.
The equilibrium reaction for the dissociation of the indicator (HIn) is :
$\text{HIn }\to \text{ I}{{\text{n}}^{-}}\text{ + }{{\text{H}}^{\text{+}}}$
The expression for pH as mentioned in the hint is :
$\text{pH = p}{{\text{K}}_{In}}\text{ + log}\frac{[(\text{conjugate ion) }\!\!]\!\!\text{ }}{[(\text{acid indicator) }\!\!]\!\!\text{ }}$
$\text{pH = p}{{\text{K}}_{In}}\text{ + log}\frac{[I{{n}^{-}}\text{ }\!\!]\!\!\text{ }}{[HIn\text{ }\!\!]\!\!\text{ }}$
2.0 = $\text{p}{{\text{K}}_{In}}$ + $\text{log }\frac{1}{1}$
$\text{p}{{\text{K}}_{In}}$ = 2.0
For $[{{H}^{+}}]\text{ = 4 x 1}{{\text{0}}^{-3}}\text{ M}$, we will calculate the pH
pH = $-\log [{{\text{H}}^{+}}]$
pH = $-\log (\text{4 x 1}{{\text{0}}^{-3}})$ = 2.4
Substituting the values, we get
$2.4\text{ = 2}\text{.0 + log}\frac{[\text{I}{{\text{n}}^{-}}]}{[\text{HIn }\!\!]\!\!\text{ }}\text{ = }\frac{100}{39.8}$
The % of indicator in unionized form in the solution = $\frac{39.8}{100+39.8}\text{ x 100}$ = 28.6%.
Therefore, the correct answer is option (C).
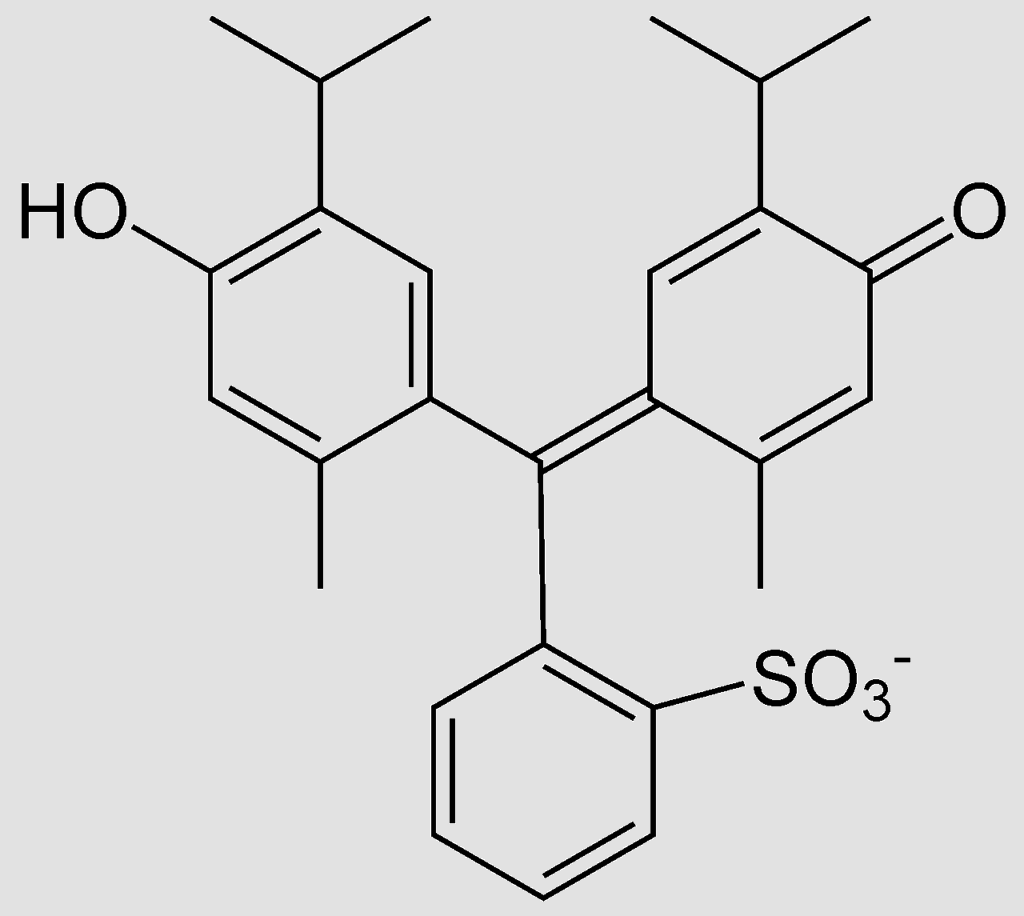
Note: The formula name for thymol blue is thymol sulfonephthalein. The structure is given below for reference:
$\text{pH = p}{{\text{K}}_{In}}\text{ + log}\frac{[(\text{conjugate ion) }\!\!]\!\!\text{ }}{[(\text{acid indicator) }\!\!]\!\!\text{ }}$
Where,
pH is potenz of hydrogen of the solution
$\text{p}{{\text{K}}_{In}}$ is dissociation constant for acid indicator
Complete step-by-step answer:
Thymol blue is a brownish-green or reddish-brown powder in crystalline form. The formula name for thymol blue is thymol sulfonephthalein. It is commonly used as a pH indicator.
Thymol blue is insoluble in water. However, it is readily soluble when alcohol is used as the solvent or very dilute alkali solutions as well.
Thymol blue changes its color from red to yellow between the range of pH 1.2 - 2.8. Then the color changes from yellow to blue between 8.0 - 9.6 pH values. It is an important component of universal indicators.
We are now going to calculate the % of indicator in unionised form.
The equilibrium reaction for the dissociation of the indicator (HIn) is :
$\text{HIn }\to \text{ I}{{\text{n}}^{-}}\text{ + }{{\text{H}}^{\text{+}}}$
The expression for pH as mentioned in the hint is :
$\text{pH = p}{{\text{K}}_{In}}\text{ + log}\frac{[(\text{conjugate ion) }\!\!]\!\!\text{ }}{[(\text{acid indicator) }\!\!]\!\!\text{ }}$
$\text{pH = p}{{\text{K}}_{In}}\text{ + log}\frac{[I{{n}^{-}}\text{ }\!\!]\!\!\text{ }}{[HIn\text{ }\!\!]\!\!\text{ }}$
2.0 = $\text{p}{{\text{K}}_{In}}$ + $\text{log }\frac{1}{1}$
$\text{p}{{\text{K}}_{In}}$ = 2.0
For $[{{H}^{+}}]\text{ = 4 x 1}{{\text{0}}^{-3}}\text{ M}$, we will calculate the pH
pH = $-\log [{{\text{H}}^{+}}]$
pH = $-\log (\text{4 x 1}{{\text{0}}^{-3}})$ = 2.4
Substituting the values, we get
$2.4\text{ = 2}\text{.0 + log}\frac{[\text{I}{{\text{n}}^{-}}]}{[\text{HIn }\!\!]\!\!\text{ }}\text{ = }\frac{100}{39.8}$
The % of indicator in unionized form in the solution = $\frac{39.8}{100+39.8}\text{ x 100}$ = 28.6%.
Therefore, the correct answer is option (C).
Note: The formula name for thymol blue is thymol sulfonephthalein. The structure is given below for reference:

Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




