
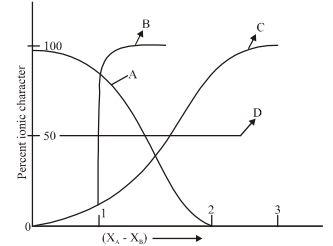
For AB bond if percent ionic character is plotted against electronegativity difference $\left( {{\chi _A} - {\chi _B}} \right)$ . The shape of the curve would look like:
A.A
B.B
C.C
D.D

Answer
566.7k+ views
Hint: We know that Electronegativity is a degree of an attraction of atoms for electrons in a bond. Electronegativity tells us the number of electrons a particular atom has. We know that the electronegativity of an atom starts from the value ranging from 0 to 4; the greater the value, the atom would be more electronegative, and the more attractive would be attraction of the electrons present in a chemical bond.
Complete answer:
We need to remember that if the electronegativity difference between the two atoms is less than ${\text{0}}{\text{.5}}$ units the type of bond is nonpolar.
We can say if the electronegativity difference between the two atoms is \[{\text{0}}{\text{.5 - 1}}{\text{.9}}\] units then the type of bond is polar covalent.
If the electronegativity difference between two atoms is greater than ${\text{1}}{\text{.9}}$ units then the type of bond is ionic.
We can give the percentage of ionic character by the equation,
Percentage of ionic character=$16\left( {{\chi _A} - {\chi _B}} \right) + 3.5{\left( {{\chi _A} - {\chi _B}} \right)^2}$
So, from the above relationship, we can observe that when $\left( {{\chi _A} - {\chi _B}} \right)$ gets increased, the percentage of ionic character would also increase. Fifty percent of ionic character takes place when the difference in electronegativity between the atoms is equal to 1.7. This is exhibited from curve C and so this is the most appropriate.
Therefore, the option (C) is correct.
If the value of electronegativity is equal to 1.7, the ionic character would be fifty percent and covalent character would be fifty percent.
If the value of electronegativity is greater than 1.7, the ionic character would be more than fifty percent and covalent character would be less than fifty percent.
If the value of electronegativity is lesser than 1.7, the ionic character would be less than fifty percent and covalent character would be greater than fifty percent.
Note:
We have to know that polarity of the bond and ionic character increases with difference in electronegativity. We can calculate the percent ionic character by calculating charge on each atom and the ionic character percent is found from the ratio of the original charge to the charge present on a single electron.
Complete answer:
We need to remember that if the electronegativity difference between the two atoms is less than ${\text{0}}{\text{.5}}$ units the type of bond is nonpolar.
We can say if the electronegativity difference between the two atoms is \[{\text{0}}{\text{.5 - 1}}{\text{.9}}\] units then the type of bond is polar covalent.
If the electronegativity difference between two atoms is greater than ${\text{1}}{\text{.9}}$ units then the type of bond is ionic.
We can give the percentage of ionic character by the equation,
Percentage of ionic character=$16\left( {{\chi _A} - {\chi _B}} \right) + 3.5{\left( {{\chi _A} - {\chi _B}} \right)^2}$
So, from the above relationship, we can observe that when $\left( {{\chi _A} - {\chi _B}} \right)$ gets increased, the percentage of ionic character would also increase. Fifty percent of ionic character takes place when the difference in electronegativity between the atoms is equal to 1.7. This is exhibited from curve C and so this is the most appropriate.
Therefore, the option (C) is correct.
If the value of electronegativity is equal to 1.7, the ionic character would be fifty percent and covalent character would be fifty percent.
If the value of electronegativity is greater than 1.7, the ionic character would be more than fifty percent and covalent character would be less than fifty percent.
If the value of electronegativity is lesser than 1.7, the ionic character would be less than fifty percent and covalent character would be greater than fifty percent.
Note:
We have to know that polarity of the bond and ionic character increases with difference in electronegativity. We can calculate the percent ionic character by calculating charge on each atom and the ionic character percent is found from the ratio of the original charge to the charge present on a single electron.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




