
For a constant volume gas thermometer, one should fill the gas at
A. Low temperature and low pressure
B. Low temperature and high pressure
C. High temperature and low pressure
D. High temperature and high pressure
Answer
604.5k+ views
Hint: In this question, we know that in a constant –volume gas thermometer we use ideal gas to fill the bulb. Now we know to achieve an ideal gas we need a very high temperature and low pressure because at these conditions the intermolecular attraction between the gas molecules is almost zero. Hence we fill the gas at high temperature and low pressure.
Complete Step-by-Step solution:
In a Constant-volume gas thermometer, there is a bulb filled with ideal gas which is attached with a mercury-filled manometer. The mercury is adjusted so that the top of the mercury column is at a 0 level and this signifies that the volume of the gas is constant as shown in figure 1.
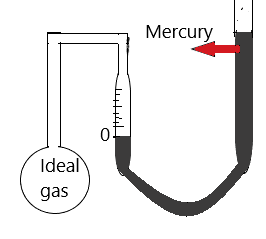
Now we measure the pressure of the gas which can be used as a measure of temperature. This is because when the temperature is decreased the pressure is decreased and similarly as the temperature is increased the pressure is increased. A Constant -volume gas thermometer provides accurate measurement over a wide range of values. As we have seen that a constant-volume gas thermometer is filled with ideal gas which can be achieved in high temperature and low pressure because in these conditions there is almost zero interaction between the gas molecules. So the correct option is C.
Additional information:
We all know that a gas thermometer works on the principle given by Charles's Law. Charles's Law states that if the temperature of a gas increases, its volume also increases. Using this concept we can easily estimate the temperature by calculating the volume of gas at a specified temperature by using the formula
$V = kT$
Where T is the temperature.
V is the volume.
k is the system constant.
Note: For these types of question we should have a clear understanding of different gas laws that is Boyle’s law, Charles’s law, Gay-Lussac’s law, combined gas law, Avogadro’s law, and Ideal gas law. We should also know about their application in real life
Complete Step-by-Step solution:
In a Constant-volume gas thermometer, there is a bulb filled with ideal gas which is attached with a mercury-filled manometer. The mercury is adjusted so that the top of the mercury column is at a 0 level and this signifies that the volume of the gas is constant as shown in figure 1.

Figure 1
Now we measure the pressure of the gas which can be used as a measure of temperature. This is because when the temperature is decreased the pressure is decreased and similarly as the temperature is increased the pressure is increased. A Constant -volume gas thermometer provides accurate measurement over a wide range of values. As we have seen that a constant-volume gas thermometer is filled with ideal gas which can be achieved in high temperature and low pressure because in these conditions there is almost zero interaction between the gas molecules. So the correct option is C.
Additional information:
We all know that a gas thermometer works on the principle given by Charles's Law. Charles's Law states that if the temperature of a gas increases, its volume also increases. Using this concept we can easily estimate the temperature by calculating the volume of gas at a specified temperature by using the formula
$V = kT$
Where T is the temperature.
V is the volume.
k is the system constant.
Note: For these types of question we should have a clear understanding of different gas laws that is Boyle’s law, Charles’s law, Gay-Lussac’s law, combined gas law, Avogadro’s law, and Ideal gas law. We should also know about their application in real life
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

The Equation xxx + 2 is Satisfied when x is Equal to Class 10 Maths

Which Country is Called "The Land of Festivals"?

What is Contraception List its four different methods class 10 biology CBSE




