
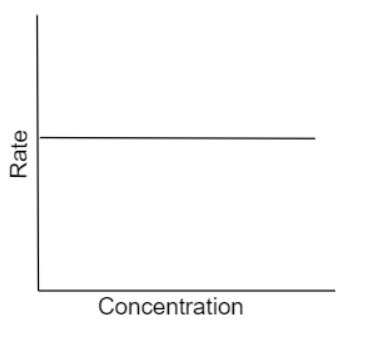
Following graph of a plot of rate of reaction vs concentration of reactant. What is the order of the reaction?

Answer
509.7k+ views
Hint: The order of a reaction is the sum of the exponents to which the concentration of the reactant terms is raised to. Thus an expression is given as $A + B \to P$. The rate of the reaction can be given as, $Rate = k{[A]^x}{[B]^y}$. The order of the reaction is $x + y$.
Complete answer:
Zero order reactions are those in which the rate doesn’t depend upon the concentration of the reactants (i.e., it is independent of the concentration of the reactants). The rate constant k shows a relationship between the rate of the reaction and the molar concentration of the reactants.
Consider a zero-order reaction $A \to P$
Where P is the product and A is the reactant. The differential rate law for the reaction can be given as:
$ - \dfrac{{d[A]}}{{dt}} = k{[A]^0} = k$-- (1)
$d[A] = - kdt$
From the above equation (1) it is clear that the rate of the reaction is independent of the reactant concentration. Thus, the graph of rate v/s reactant concentration will be a straight line parallel to the x- axis. Hence the given plot is for a zero-order reaction
The rate law for a zero-order reaction is given as:
$[A] = {[A]_0} - kt$
Where ${[A]_o}$ is the initial concentration of the reactant at $t = 0$and [A] is the final concentration of the reaction at time ‘t’. t is the duration of the reaction and k is the rate constant.
Note:
Rate of the reaction indicates how fast does reactant convert into products. In first order reaction the rate is dependent on the reactant concentration. In second order reaction the rate depends on both the reactant and the substrate concentration. A simple example of zero order reaction is iodization of acetone.
Complete answer:
Zero order reactions are those in which the rate doesn’t depend upon the concentration of the reactants (i.e., it is independent of the concentration of the reactants). The rate constant k shows a relationship between the rate of the reaction and the molar concentration of the reactants.
Consider a zero-order reaction $A \to P$
Where P is the product and A is the reactant. The differential rate law for the reaction can be given as:
$ - \dfrac{{d[A]}}{{dt}} = k{[A]^0} = k$-- (1)
$d[A] = - kdt$
From the above equation (1) it is clear that the rate of the reaction is independent of the reactant concentration. Thus, the graph of rate v/s reactant concentration will be a straight line parallel to the x- axis. Hence the given plot is for a zero-order reaction
The rate law for a zero-order reaction is given as:
$[A] = {[A]_0} - kt$
Where ${[A]_o}$ is the initial concentration of the reactant at $t = 0$and [A] is the final concentration of the reaction at time ‘t’. t is the duration of the reaction and k is the rate constant.
Note:
Rate of the reaction indicates how fast does reactant convert into products. In first order reaction the rate is dependent on the reactant concentration. In second order reaction the rate depends on both the reactant and the substrate concentration. A simple example of zero order reaction is iodization of acetone.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




