
How is fluorine prepared by Whytlaw-Gray method? Draw a diagram.
Answer
597.3k+ views
Hint: The Whytlaw-Gray method is based on the principle that $KH{{F}_{2}}$ which is dry, anhydrous and pure in nature is used, is electrolysed in the molten state. Here, we can see that the fluorine is liberated at cathode and hydrogen is liberated at anode.
Complete step by step answer:
- Electrolysis of fused potassium hydrogen fluoride is carried out in electric preheated copper cells which also act as cathode. We can see the diagram of whytlaw -gray method:
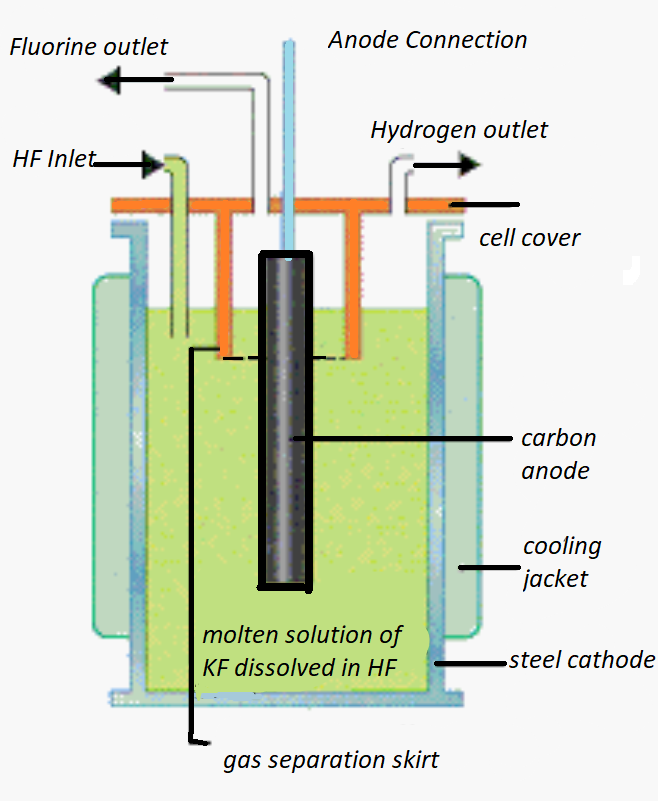
- We can see that on fusion this reaction takes place:
\[\begin{align}
& 2KH{{F}_{2}}\to 2KF+2HF \\
& 2KF\to 2{{K}^{+}}+2{{F}^{-}} \\
& \\
\end{align}\]
Which on further electrolysis, at anode, following reaction takes place:
\[2{{F}^{-}}\to {{F}_{2}}+2{{e}^{-}}\]
So, this is our desired fluorine.
And the reaction that takes place at cathode is:
\[\begin{align}
& 2K+2{{e}^{-}}\to 2K \\
& 2K+2HF\to 2KF+{{H}_{2}}\uparrow \\
\end{align}\]
- If we see its diagram , we have a heated copper cell, coils, solution which is fused $KH{{F}_{2}}$ and an anode of graphite, copper diaphragm, fluorspar stopper etc.
- There is a cylindrical vessel, the vertical walls of the vessel minimise creeping of the electrolyte. There is also diaphragm present in between electrodes, which basically prevents the mixing of hydrogen and fluorine.
- There are electrodes present, we can see in the diagram that graphite rod acts as anode and copper vessel acts as cathode.
- We can see that anode and cathode are separated by a diaphragm. The anode is found to be insulated from the diaphragm cell by a paste of fluorspar. The upper part is non-porous that leads to a delivery tube for the${{F}_{2}}$ gas.
- It is also seen that, in the electrolysis process, the temperature is also maintained to about 523K, there is $KH{{F}_{2}}$ electrolyte used which is dry, pure and anhydrous in nature.
- There is an outlet for fluorine gas present in inner copper diaphragm, and the outer copper vessel has an outlet for the hydrogen gas.
- And finally, the fluorine gas is collected in copper cylinders, which is then compressed and stored in Cu-Ni cylinders.
Note: - We can say that this method is more efficient than other processes and here the current efficiency is as high as 80 percent. And also this process is a continuous process.
Complete step by step answer:
- Electrolysis of fused potassium hydrogen fluoride is carried out in electric preheated copper cells which also act as cathode. We can see the diagram of whytlaw -gray method:

- We can see that on fusion this reaction takes place:
\[\begin{align}
& 2KH{{F}_{2}}\to 2KF+2HF \\
& 2KF\to 2{{K}^{+}}+2{{F}^{-}} \\
& \\
\end{align}\]
Which on further electrolysis, at anode, following reaction takes place:
\[2{{F}^{-}}\to {{F}_{2}}+2{{e}^{-}}\]
So, this is our desired fluorine.
And the reaction that takes place at cathode is:
\[\begin{align}
& 2K+2{{e}^{-}}\to 2K \\
& 2K+2HF\to 2KF+{{H}_{2}}\uparrow \\
\end{align}\]
- If we see its diagram , we have a heated copper cell, coils, solution which is fused $KH{{F}_{2}}$ and an anode of graphite, copper diaphragm, fluorspar stopper etc.
- There is a cylindrical vessel, the vertical walls of the vessel minimise creeping of the electrolyte. There is also diaphragm present in between electrodes, which basically prevents the mixing of hydrogen and fluorine.
- There are electrodes present, we can see in the diagram that graphite rod acts as anode and copper vessel acts as cathode.
- We can see that anode and cathode are separated by a diaphragm. The anode is found to be insulated from the diaphragm cell by a paste of fluorspar. The upper part is non-porous that leads to a delivery tube for the${{F}_{2}}$ gas.
- It is also seen that, in the electrolysis process, the temperature is also maintained to about 523K, there is $KH{{F}_{2}}$ electrolyte used which is dry, pure and anhydrous in nature.
- There is an outlet for fluorine gas present in inner copper diaphragm, and the outer copper vessel has an outlet for the hydrogen gas.
- And finally, the fluorine gas is collected in copper cylinders, which is then compressed and stored in Cu-Ni cylinders.
Note: - We can say that this method is more efficient than other processes and here the current efficiency is as high as 80 percent. And also this process is a continuous process.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




