
Fluorine exhibits only -1 oxidation state while iodine exhibits oxidation states of -1, +1, +3, +5 and +7. This is due to:
(A) fluorine being a gas.
(B) available d-orbitals in iodine.
(C) non-availability of d-orbitals in iodine.
(D) none of the above.
Answer
573k+ views
Hint: Fluorine and Iodine belongs to Group 17. They are commonly called as Halogens. Halogens are highly reactive nonmetals and also known as p-block elements.
Complete answer:
Oxidation state means degree of oxidation for an atom in a chemical compound. Oxidation state represented by an integer, which can be positive, negative or zero.
Fluorine and iodine are group 17 members.
There electronic configuration is
F = Atomic number = 9 = $1{s^2}2{s^2}2{p^5} = [He]2{s^2}2{p^5}$
I = Atomic number = 53 = $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}4{d^{10}}5{s^2}5{p^6}$
$ = [Kr]4{d^{10}}5{d^2}5{p^6}$
Ground state Electronic configuration of fluorine is
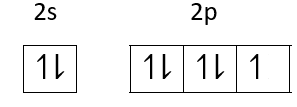
Fluorine is the most electronegative atom so it accepts electrons very easily and shows only -1 oxidation state.
Ground state electronic configuration of iodine is shown in the diagram below.
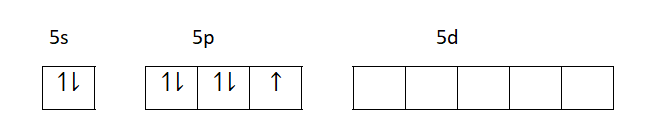
First excited state
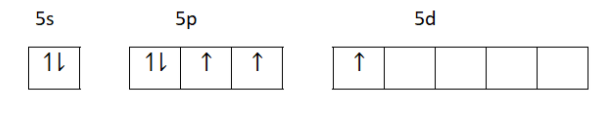
Second excited state

Third excited state
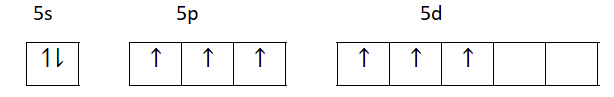
Therefore, iodine shows +1, +3, +5, +7 oxidation state apart from oxidation state -1 because of the presence of empty vacant d- orbitals which are not present in fluorine. So Fluorine does not show any higher oxidation state.
Note: Positive oxidation state is possible by excitation of outer s and p-orbitals into d-orbitals so that 3, 5 or 7 unpaired electrons are easily available for bonding. Elements which do not have vacant d orbitals can’t show higher oxidation state.
Complete answer:
Oxidation state means degree of oxidation for an atom in a chemical compound. Oxidation state represented by an integer, which can be positive, negative or zero.
Fluorine and iodine are group 17 members.
There electronic configuration is
F = Atomic number = 9 = $1{s^2}2{s^2}2{p^5} = [He]2{s^2}2{p^5}$
I = Atomic number = 53 = $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}4{d^{10}}5{s^2}5{p^6}$
$ = [Kr]4{d^{10}}5{d^2}5{p^6}$
Ground state Electronic configuration of fluorine is
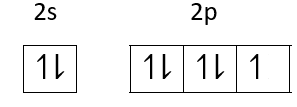
Fluorine is the most electronegative atom so it accepts electrons very easily and shows only -1 oxidation state.
Ground state electronic configuration of iodine is shown in the diagram below.

First excited state

Second excited state

Third excited state

Therefore, iodine shows +1, +3, +5, +7 oxidation state apart from oxidation state -1 because of the presence of empty vacant d- orbitals which are not present in fluorine. So Fluorine does not show any higher oxidation state.
Note: Positive oxidation state is possible by excitation of outer s and p-orbitals into d-orbitals so that 3, 5 or 7 unpaired electrons are easily available for bonding. Elements which do not have vacant d orbitals can’t show higher oxidation state.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




