
Find the total number of isomers of ${C_7}{H_{14}}$ (only 5-membered ring)
Answer
565.2k+ views
:Hint: Isomers are structures of a compound with the same number of atoms but they are structures with different positions of the atoms and the new structure has different chemical and physical properties. They are very important in determining the right position of an atom in a structure to obtain the desired product.
Complete answer:
As we know that isomers are structures of a compound which have the same number of atoms but have different arrangements of atoms which result in different chemical and physical properties of the isomeric structures. This formation of isomers is known as isomerism. These differ in terms of structure or the arrangement of atoms which is known as structural isomerism. Structural isomers are of three types which depend on the chain of the structures, presence of functional group and the position of various atoms in the isomeric structures. The chain isomers have the same molecular formula but as their names suggest they differ in terms of arrangement of atoms and branches, similarly functional isomers have the same molecular formula but different functional groups attached in different isomeric structures.
Stereoisomers are those isomers which have the same molecular formula but different arrangements of atoms in two dimensional and three dimensional planes. Geometric isomers are those isomers in which the structures differ due to different symmetry.
So, for the given question ${C_7}{H_{14}}$ has 6 isomers including stereoisomers and they are 5-membered rings.
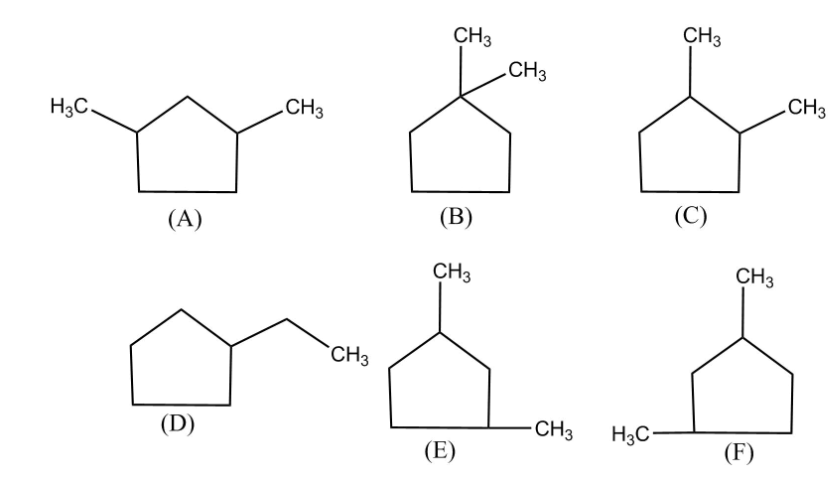
Thus, we see the six possible isomers which are 5-membered ring structures.
Isomers are very helpful in determining the right position of each atom in a compound which helps us to obtain the desired product as the change in atoms can result in an isomer which may have undesired chemical and physical properties.
Note:
Isomerism has been a very powerful and important tool in determining the most effective drug as it helps to obtain those structures which have desired chemical and physical properties. Branched isomers have lower melting and boiling points because of the presence of stronger Van der waals forces in the unbranched or straight chains.
Complete answer:
As we know that isomers are structures of a compound which have the same number of atoms but have different arrangements of atoms which result in different chemical and physical properties of the isomeric structures. This formation of isomers is known as isomerism. These differ in terms of structure or the arrangement of atoms which is known as structural isomerism. Structural isomers are of three types which depend on the chain of the structures, presence of functional group and the position of various atoms in the isomeric structures. The chain isomers have the same molecular formula but as their names suggest they differ in terms of arrangement of atoms and branches, similarly functional isomers have the same molecular formula but different functional groups attached in different isomeric structures.
Stereoisomers are those isomers which have the same molecular formula but different arrangements of atoms in two dimensional and three dimensional planes. Geometric isomers are those isomers in which the structures differ due to different symmetry.
So, for the given question ${C_7}{H_{14}}$ has 6 isomers including stereoisomers and they are 5-membered rings.

Thus, we see the six possible isomers which are 5-membered ring structures.
Isomers are very helpful in determining the right position of each atom in a compound which helps us to obtain the desired product as the change in atoms can result in an isomer which may have undesired chemical and physical properties.
Note:
Isomerism has been a very powerful and important tool in determining the most effective drug as it helps to obtain those structures which have desired chemical and physical properties. Branched isomers have lower melting and boiling points because of the presence of stronger Van der waals forces in the unbranched or straight chains.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




