
Find the oxidation number of Br in \[B{{r}_{3}}{{O}_{8}}\].
Answer
526.3k+ views
Hint: It has a fractional oxidation number. This compound has two types of oxidation state on bromine because of its structure. These are combined to form a single oxidation state.
Complete step by step answer:
The oxidation number of an element is defined as the charge the atom of an element has in its ion or appears to have when it is in a combined form with other atoms. The oxidation number is also known as the oxidation state.
In neutral compounds, the sum state of all the atoms is zero.
In complexion, the sum of the oxidation state of all atoms in the ion is equal to the charge on the ion.
For \[B{{r}_{3}}{{O}_{8}}\] let us first draw the structure:
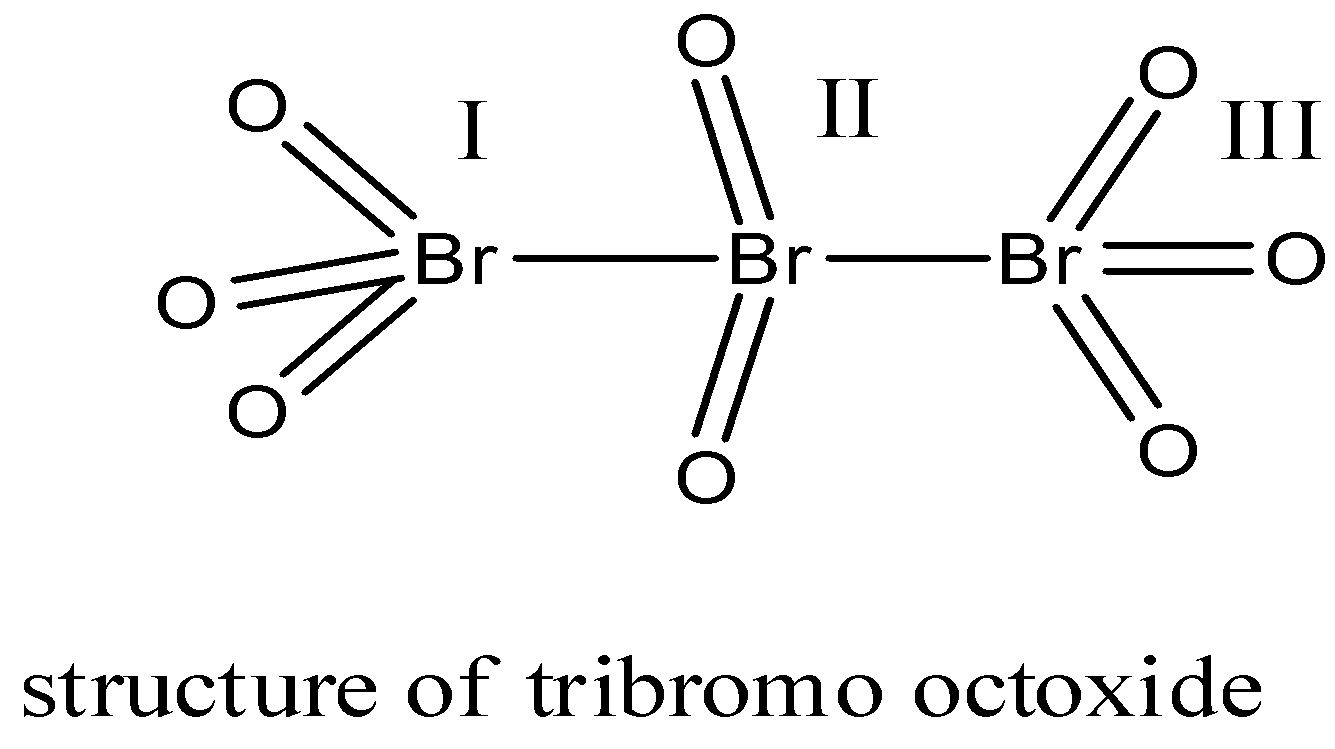
In this structure, there are 3 bromine atoms which form a bridge-like structure. There are 2 terminal bromine atoms and one central atom. Both the terminal atoms are joined to 3 oxygen atoms.
So, the oxidation state of both the terminal bromine atoms will be = x + 3(-2) = x – 6.
X = +6.
The central atom is joined to 2 oxygen atoms.
So, the oxidation state of the central bromine will be = x + 2(-2) = x – 4
X = +4.
The\[B{{r}_{3}}{{O}_{8}}\] compound has two types of oxidation states i.e. +6 and +4.
But for the overall compound we take the average of all 3 oxidation state:
\[\dfrac{6+6+4}{3}=\dfrac{16}{3}\]
\[\begin{align}
& x+\dfrac{16}{3}=0 \\
& \\
\end{align}\]
\[x=-\dfrac{16}{3}\]
Hence, the oxidation of bromine is -16/3.
Note: In this question, you cannot easily write the equation directly to solve the oxidation number. This will answer 16/3, which is wrong. The structure should be considered in these types of questions. This compound has a fractional oxidation state.
Complete step by step answer:
The oxidation number of an element is defined as the charge the atom of an element has in its ion or appears to have when it is in a combined form with other atoms. The oxidation number is also known as the oxidation state.
In neutral compounds, the sum state of all the atoms is zero.
In complexion, the sum of the oxidation state of all atoms in the ion is equal to the charge on the ion.
For \[B{{r}_{3}}{{O}_{8}}\] let us first draw the structure:

In this structure, there are 3 bromine atoms which form a bridge-like structure. There are 2 terminal bromine atoms and one central atom. Both the terminal atoms are joined to 3 oxygen atoms.
So, the oxidation state of both the terminal bromine atoms will be = x + 3(-2) = x – 6.
X = +6.
The central atom is joined to 2 oxygen atoms.
So, the oxidation state of the central bromine will be = x + 2(-2) = x – 4
X = +4.
The\[B{{r}_{3}}{{O}_{8}}\] compound has two types of oxidation states i.e. +6 and +4.
But for the overall compound we take the average of all 3 oxidation state:
\[\dfrac{6+6+4}{3}=\dfrac{16}{3}\]
\[\begin{align}
& x+\dfrac{16}{3}=0 \\
& \\
\end{align}\]
\[x=-\dfrac{16}{3}\]
Hence, the oxidation of bromine is -16/3.
Note: In this question, you cannot easily write the equation directly to solve the oxidation number. This will answer 16/3, which is wrong. The structure should be considered in these types of questions. This compound has a fractional oxidation state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




