
Fill in the blanks.
The electrolysis of potassium fumarate gives _____.
Answer
579.9k+ views
Hint:The electrolysis of potassium fumarate is an important and commonly used method for the preparation of a key chemical which is used in the production of several organic compounds of great industrial importance.
Complete step by step answer:
Acetylene is an unsaturated alkyne because the two carbons of acetylene are bonded together by a triple bond. It is also named ethyne as it is an alkyne containing 2 carbon atoms. Acetylene is used in the manufacture of synthetic fibres, rubber, plastics, acetaldehyde, acetic acid and ethyl alcohol. Because of this, many special methods have been used for its preparation.
One such method is the electrolysis of aqueous solution of sodium or potassium fumarate. The structure of potassium fumarate is:
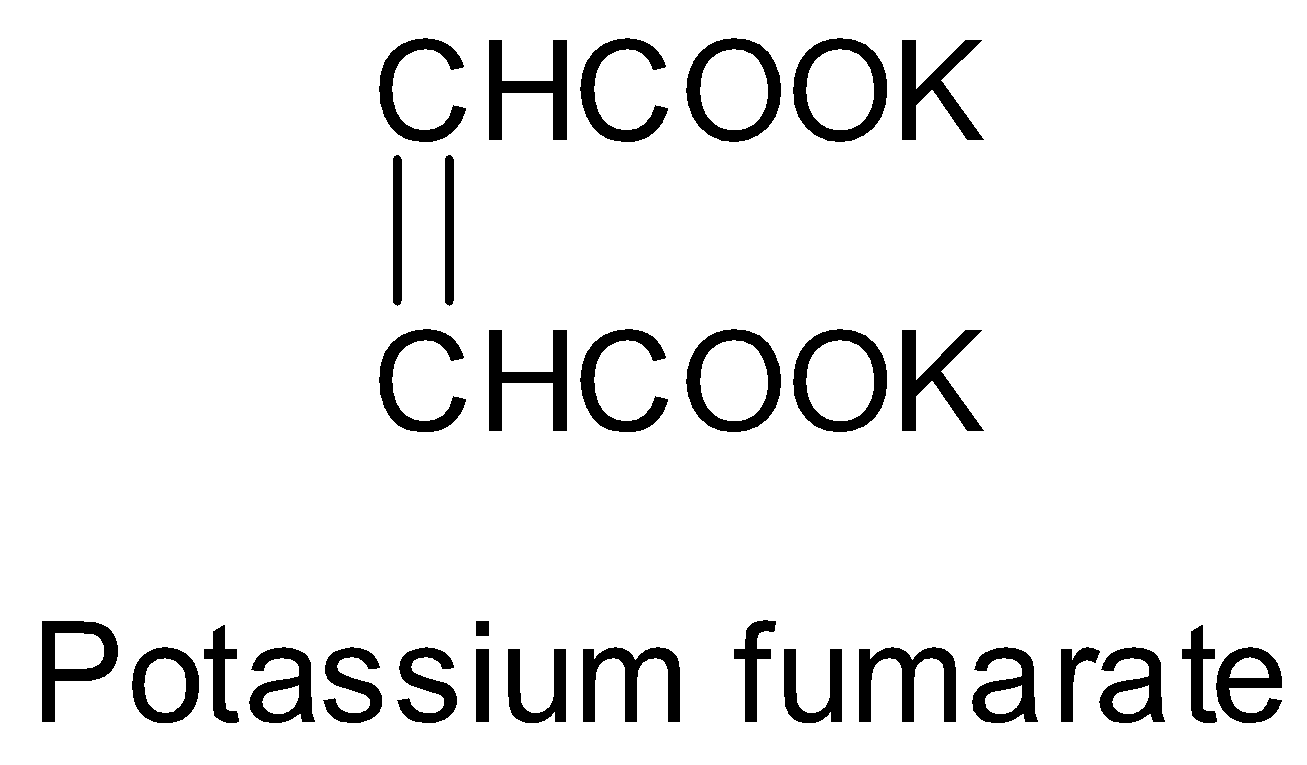
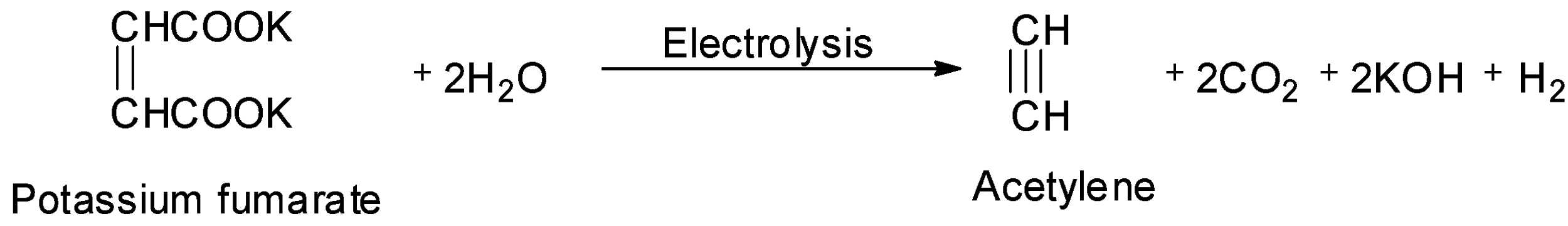
Thus, the electrolysis of potassium fumarate will give acetylene. Along with acetylene, carbon dioxide, potassium hydroxide and hydrogen gas is also produced. The reaction of the electrolysis of aqueous solution of potassium fumarate is shown below.
Note:
Another commonly used method for the preparation of acetylene is by the action of water on calcium carbide. The reaction is:
$\mathop {{\text{Ca}}{{\text{C}}_{\text{2}}}}\limits_{{\text{Calcium Carbide}}} {\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}} \to \mathop {{\text{HC}} \equiv {\text{HC}}}\limits_{{\text{Acetylene}}} {\text{ + Ca}}{\left( {{\text{OH}}} \right)_{\text{2}}}$
The calcium carbide required for this purpose is itself obtained by heating coke and calcium oxide in an electric furnace.
${\text{CaO + 3C}}\xrightarrow{{{\text{2273K}}}}{\text{Ca}}{{\text{C}}_{\text{2}}}{\text{ + CO}}$
This method is suitable for the laboratory as well as for the large scale production of acetylene. The gas produced here is collected by the downward displacement of water. The gas obtained by the above method usually contains the impurities of hydrogen sulphide and phosphine which are removed by the bubbling of the gas through acidified copper sulphate solution.
Complete step by step answer:
Acetylene is an unsaturated alkyne because the two carbons of acetylene are bonded together by a triple bond. It is also named ethyne as it is an alkyne containing 2 carbon atoms. Acetylene is used in the manufacture of synthetic fibres, rubber, plastics, acetaldehyde, acetic acid and ethyl alcohol. Because of this, many special methods have been used for its preparation.
One such method is the electrolysis of aqueous solution of sodium or potassium fumarate. The structure of potassium fumarate is:

Thus, the electrolysis of potassium fumarate will give acetylene. Along with acetylene, carbon dioxide, potassium hydroxide and hydrogen gas is also produced. The reaction of the electrolysis of aqueous solution of potassium fumarate is shown below.

Note:
Another commonly used method for the preparation of acetylene is by the action of water on calcium carbide. The reaction is:
$\mathop {{\text{Ca}}{{\text{C}}_{\text{2}}}}\limits_{{\text{Calcium Carbide}}} {\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}} \to \mathop {{\text{HC}} \equiv {\text{HC}}}\limits_{{\text{Acetylene}}} {\text{ + Ca}}{\left( {{\text{OH}}} \right)_{\text{2}}}$
The calcium carbide required for this purpose is itself obtained by heating coke and calcium oxide in an electric furnace.
${\text{CaO + 3C}}\xrightarrow{{{\text{2273K}}}}{\text{Ca}}{{\text{C}}_{\text{2}}}{\text{ + CO}}$
This method is suitable for the laboratory as well as for the large scale production of acetylene. The gas produced here is collected by the downward displacement of water. The gas obtained by the above method usually contains the impurities of hydrogen sulphide and phosphine which are removed by the bubbling of the gas through acidified copper sulphate solution.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




