
Explain why propanol has higher boiling point than that of the butane?
Answer
580.2k+ views
Hint: When two compounds have similar molecular weight, then, they should have similar boiling point. However, in one compound, if additional interactions are present, then it will have higher boiling point. Identify the additional interactions present in propanol.
Complete Step by step answer: Write the chemical structure of propanol and butane.
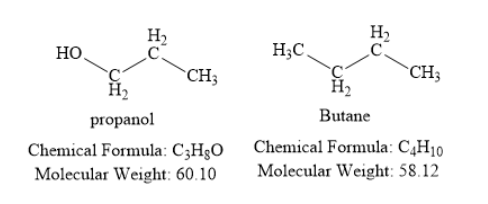
Propanol contains three carbon atoms, eight hydrogen atoms and one oxygen atom. The oxygen atom is present in the form of hydroxyl group. Thus, propanol is an alcohol.
Butane is a hydrocarbon. It contains four carbon atoms and ten hydrogen atoms.
The molecular weight (in grams per mole) of propanol is 60.10. The molecular weight (in grams per mole) of butane is 58.12. Thus, both propanol and butane have similar molecular weight. Hence, you should expect that the boiling point of propanol is similar to the boiling point of butane. However, in reality, propanol has much higher boiling point than butane. This is due to the presence of intermolecular hydrogen bonding.
In propanol, the electronegative oxygen atom of hydroxyl group has one hydrogen atom attached to it. Due to this intermolecular hydrogen bonds are formed in propanol. This leads to molecular association. To break this molecular association, a huge amount of energy is needed. This increases the boiling point of propanol.
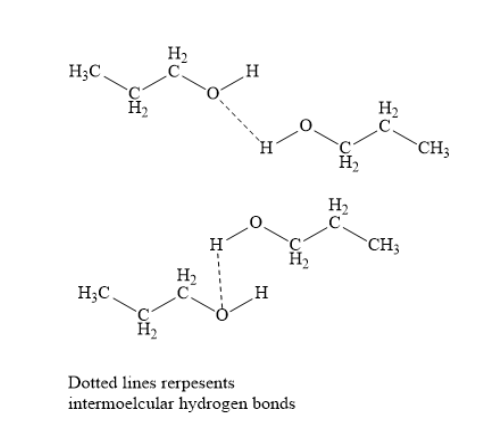
In butane, hydrogen bonds are not formed. Hence, butane has lower boiling point. Thus, propanol has higher boiling point than that of the butane.
Note: A hydrogen bond is formed when hydrogen atom is attached to electronegative nitrogen, oxygen or fluorine atom. If hydrogen atom is not attached to electronegative nitrogen, oxygen or fluorine atom, then hydrogen bonding formation is not possible.
Complete Step by step answer: Write the chemical structure of propanol and butane.

Propanol contains three carbon atoms, eight hydrogen atoms and one oxygen atom. The oxygen atom is present in the form of hydroxyl group. Thus, propanol is an alcohol.
Butane is a hydrocarbon. It contains four carbon atoms and ten hydrogen atoms.
The molecular weight (in grams per mole) of propanol is 60.10. The molecular weight (in grams per mole) of butane is 58.12. Thus, both propanol and butane have similar molecular weight. Hence, you should expect that the boiling point of propanol is similar to the boiling point of butane. However, in reality, propanol has much higher boiling point than butane. This is due to the presence of intermolecular hydrogen bonding.
In propanol, the electronegative oxygen atom of hydroxyl group has one hydrogen atom attached to it. Due to this intermolecular hydrogen bonds are formed in propanol. This leads to molecular association. To break this molecular association, a huge amount of energy is needed. This increases the boiling point of propanol.

In butane, hydrogen bonds are not formed. Hence, butane has lower boiling point. Thus, propanol has higher boiling point than that of the butane.
Note: A hydrogen bond is formed when hydrogen atom is attached to electronegative nitrogen, oxygen or fluorine atom. If hydrogen atom is not attached to electronegative nitrogen, oxygen or fluorine atom, then hydrogen bonding formation is not possible.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




