
Explain the method of preparation of hydrogen gas in the laboratory with chemical equation and diagram?
Answer
598.8k+ views
Hint: The laboratory preparation of hydrogen gas involves the reaction of dilute hydrochloric acid on zinc pieces. We need a $500$ ml flask, a delivery tube, a test tube and a thistle funnel to perform this experiment.
Complete step by step solution:
> First of all we will take a few grams of zinc pieces and put them in the flask. Now add dilute Hydrochloric acid to zinc granules with the help of thistle funnel. After addition of dilute $HCl$ to the zinc pieces hydrogen gas will be produced automatically and this gas will be collected with the help of a delivery tube in a container full of water. Hence hydrogen gas is collected by the downward displacement of water.
Chemical equation: $Zn + 2HCl \to ZnC{l_2} + {H_2}$
> Uses and properties of hydrogen gas- Hydrogen gas is used in rocket fuel and in production of other compounds. It is used in welding, hydrogenation of fats and oils. Hydrogen is a diatomic gas. It is a tasteless, flammable and odourless substance .It can be stored in compressed form as a liquid. With the change of temperature, solubility of hydrogen gas in water does not affect it very much.
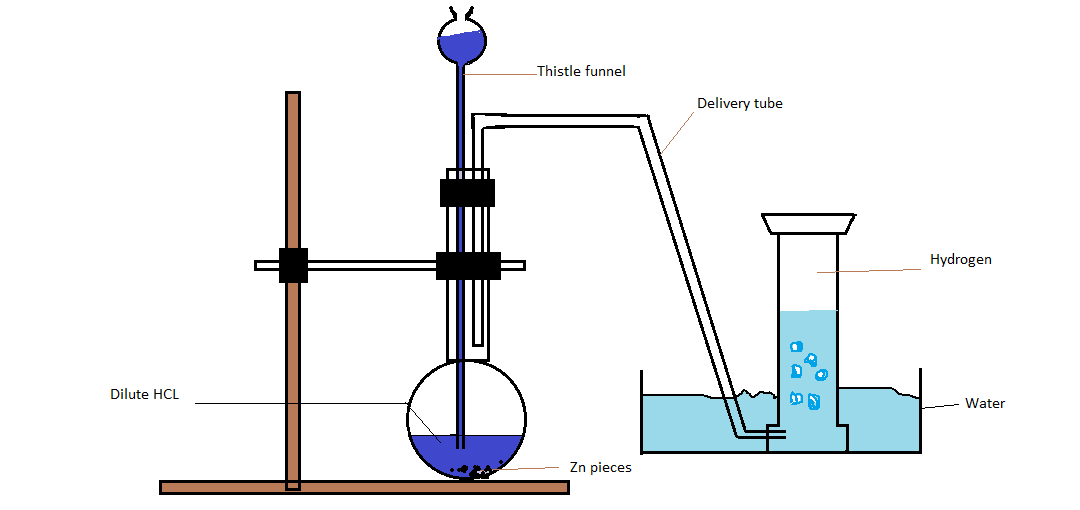
Laboratory method of preparation of hydrogen gas
Note: Hence hydrogen gas can be prepared in the laboratory with the help of conical flask ,fitted with the delivery tube. In the flask a few grams of granulated zinc are taken then dilute hydrochloric acid is added to the flask and liberated hydrogen gas is collected in an inverted test tube placed in water. Hydrogen gas obtained by this procedure contains different impurities like ${H_2}S,N{O_2},C{O_2}$ and moisture etc.
Complete step by step solution:
> First of all we will take a few grams of zinc pieces and put them in the flask. Now add dilute Hydrochloric acid to zinc granules with the help of thistle funnel. After addition of dilute $HCl$ to the zinc pieces hydrogen gas will be produced automatically and this gas will be collected with the help of a delivery tube in a container full of water. Hence hydrogen gas is collected by the downward displacement of water.
Chemical equation: $Zn + 2HCl \to ZnC{l_2} + {H_2}$
> Uses and properties of hydrogen gas- Hydrogen gas is used in rocket fuel and in production of other compounds. It is used in welding, hydrogenation of fats and oils. Hydrogen is a diatomic gas. It is a tasteless, flammable and odourless substance .It can be stored in compressed form as a liquid. With the change of temperature, solubility of hydrogen gas in water does not affect it very much.

Laboratory method of preparation of hydrogen gas
Note: Hence hydrogen gas can be prepared in the laboratory with the help of conical flask ,fitted with the delivery tube. In the flask a few grams of granulated zinc are taken then dilute hydrochloric acid is added to the flask and liberated hydrogen gas is collected in an inverted test tube placed in water. Hydrogen gas obtained by this procedure contains different impurities like ${H_2}S,N{O_2},C{O_2}$ and moisture etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




