
Explain the Haworth projection of glucose.
Answer
548.1k+ views
Hint: The Haworth projection is simply the three-dimensional perspective of seeing the structure of a molecule represented as the cyclic structure of monosaccharides. More commonly, Fischer projection is converted into the Haworth projection and chair conformation and vice versa.
Complete step-by-step answer:
Let us discuss the concept of Haworth projection in detail;
Haworth projection:
Biochemists often draw the structure of sugars in the form of Fischer projections and Haworth projections. Fischer projection shows the structure in the form of open chains. In this, the carbon atoms of a sugar molecule are connected vertically by solid lines whereas, the carbon-oxygen and carbon-hydrogen is connected by horizontal bonding within them.
This Fischer projection is converted into Haworth projection just by representing itself in the cyclic form. This conversion takes place in some number of steps as described below (specifically for glucose);
We need to rotate the bonds around C-5 clockwise.
Next draw the hexagon omitting the line between OH and CHO.
Further, add $C{{H}_{2}}$ and OH groups to the structure.
Now, connect the line we omitted in step 2 (forming a pyran).
Finally, add the H groups opposite to the OH groups.
Now, as the glucose can exists in two forms (due to the position of H and OH groups on the anomeric carbon); we have shown that as below:
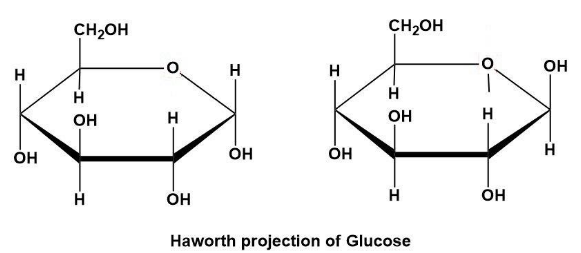
Note: Do note that the Haworth projection can further be converted into chair conformation. But for ease of demonstration many chemists prefer Fischer projection or Haworth projection in general instead of chair conformation.
Complete step-by-step answer:
Let us discuss the concept of Haworth projection in detail;
Haworth projection:
Biochemists often draw the structure of sugars in the form of Fischer projections and Haworth projections. Fischer projection shows the structure in the form of open chains. In this, the carbon atoms of a sugar molecule are connected vertically by solid lines whereas, the carbon-oxygen and carbon-hydrogen is connected by horizontal bonding within them.
This Fischer projection is converted into Haworth projection just by representing itself in the cyclic form. This conversion takes place in some number of steps as described below (specifically for glucose);
We need to rotate the bonds around C-5 clockwise.
Next draw the hexagon omitting the line between OH and CHO.
Further, add $C{{H}_{2}}$ and OH groups to the structure.
Now, connect the line we omitted in step 2 (forming a pyran).
Finally, add the H groups opposite to the OH groups.
Now, as the glucose can exists in two forms (due to the position of H and OH groups on the anomeric carbon); we have shown that as below:

Note: Do note that the Haworth projection can further be converted into chair conformation. But for ease of demonstration many chemists prefer Fischer projection or Haworth projection in general instead of chair conformation.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




