
Explain the Haber's process of manufacturing Ammonia with a neat diagram.
Answer
586.8k+ views
Hint: The Haber process also known as Haber–Bosch process is a process of an artificial nitrogen fixation and is considered to be the main industrial phenomenon for ammonia production. The process is named after its German inventors namely Carl Bosch and Fritz Haber.
Complete step by step answer:
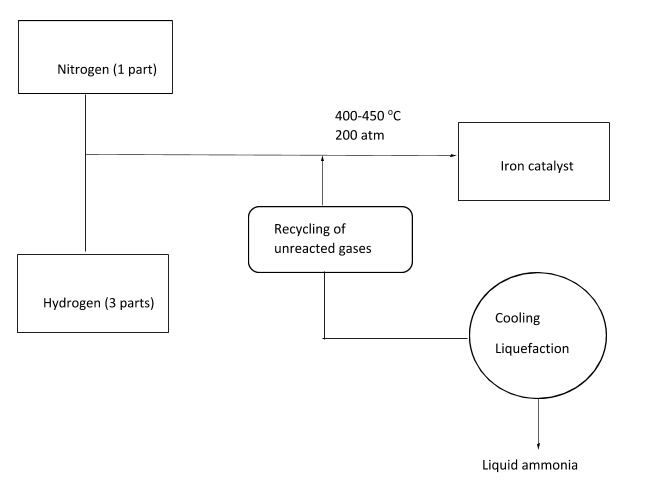
The Haber’s process combines nitrogen with hydrogen in the ratio of 1:3 in order to produce ammonia. Nitrogen is supplied from air while the major source for hydrogen is methane from the natural gas. Reversible reaction takes place and the generation of ammonia is an exothermic reaction. This reaction taking place in the reactor at temperature of 400-450 oC and 200 atm pressure can be written in the following manner:
\[{N_2}\left( g \right){\text{ }} + {\text{ }}{H_2}\left( g \right){\text{ }} \rightleftharpoons {\text{ }}2N{H_3}\left( g \right)\]
In this reaction, iron is generally utilized as a catalyst. At each stage of the passage of gases through a reactor, only around 15% of nitrogen as well as hydrogen are converted to ammonia. By continuously recycling the unreacted nitrogen as well as hydrogen, 98% of ammonia can be obtained. In the reaction, nitrogen can be attained by the process of liquefaction by removing nitrogen from soil. On the other hand, hydrogen can be attained by the process of steam reforming from the natural gas.
$C{H_4}(g) + {H_2}O \to {H_2}(g) + CO(g)$
The Haber process can be better understood with the following diagram:
Note:
The generation of ammonia through the Haber process is the most energy-intensive commodity chemical which is responsible for almost 1%–2% of consumption of global energy and 1.44% of \[C{O_2}\] emissions.
Complete step by step answer:
The Haber’s process combines nitrogen with hydrogen in the ratio of 1:3 in order to produce ammonia. Nitrogen is supplied from air while the major source for hydrogen is methane from the natural gas. Reversible reaction takes place and the generation of ammonia is an exothermic reaction. This reaction taking place in the reactor at temperature of 400-450 oC and 200 atm pressure can be written in the following manner:
\[{N_2}\left( g \right){\text{ }} + {\text{ }}{H_2}\left( g \right){\text{ }} \rightleftharpoons {\text{ }}2N{H_3}\left( g \right)\]
In this reaction, iron is generally utilized as a catalyst. At each stage of the passage of gases through a reactor, only around 15% of nitrogen as well as hydrogen are converted to ammonia. By continuously recycling the unreacted nitrogen as well as hydrogen, 98% of ammonia can be obtained. In the reaction, nitrogen can be attained by the process of liquefaction by removing nitrogen from soil. On the other hand, hydrogen can be attained by the process of steam reforming from the natural gas.
$C{H_4}(g) + {H_2}O \to {H_2}(g) + CO(g)$
The Haber process can be better understood with the following diagram:

Note:
The generation of ammonia through the Haber process is the most energy-intensive commodity chemical which is responsible for almost 1%–2% of consumption of global energy and 1.44% of \[C{O_2}\] emissions.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




