
Explain the following named reaction:
i)Sandmeyer reaction.
ii)Gattermann reaction.
Answer
597k+ views
Hint: The name reactions are those reactions which are named after its discoverers, or developers. The above name reactions are used for the formation of aryl halide from diazonium salts.
Complete step by step answer:
Sandmeyer reaction: In this reaction when diazonium salts are treated with $CuCl$ dissolved in $HCl$ or $CuBr$ dissolved in $HBr$ then formation of aryl chloride or aryl bromide takes place.
The sandmeyer reaction is written as:
In the first step we form nitrous acid from sodium nitrite.
$NaN{O_2} + HCl(dil) \to HONO + NaCl$
Then nitrous acid reacts with aniline to form diazonium salt.
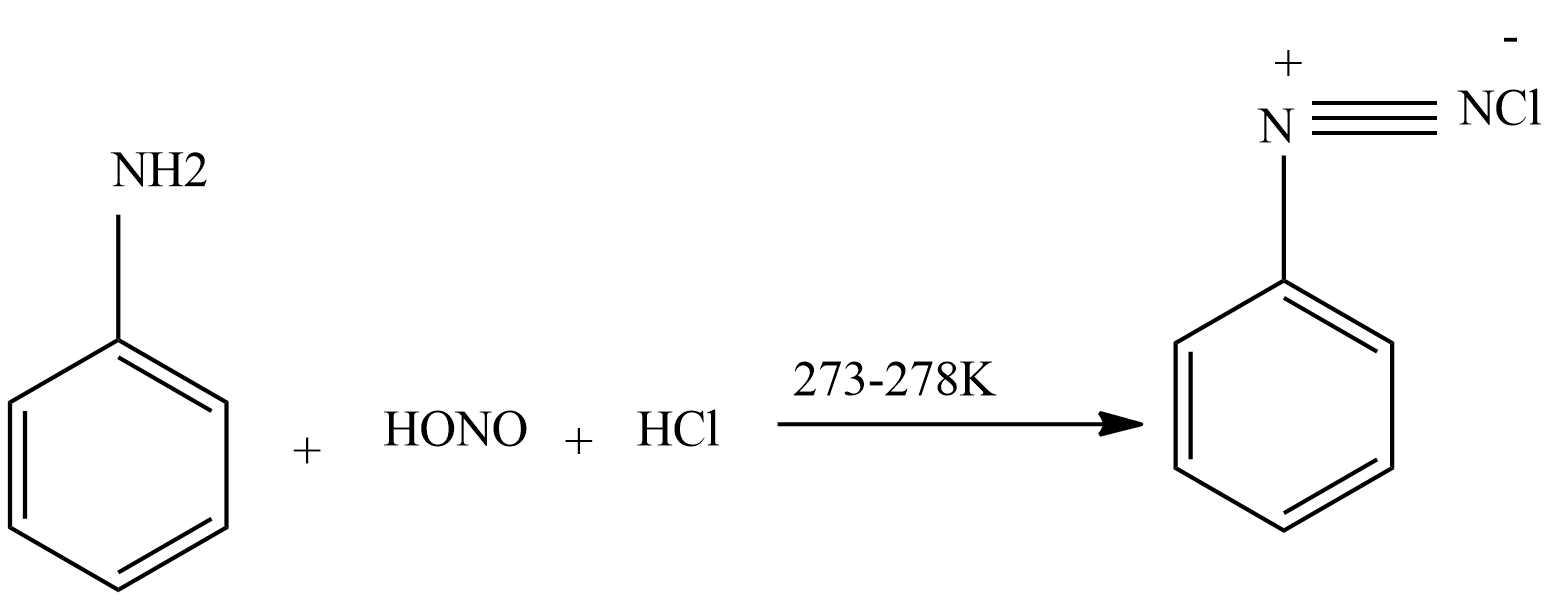
Then diazonium salt reacts with $CuCl$ dissolved in $HCl$ to form chloroarenes.
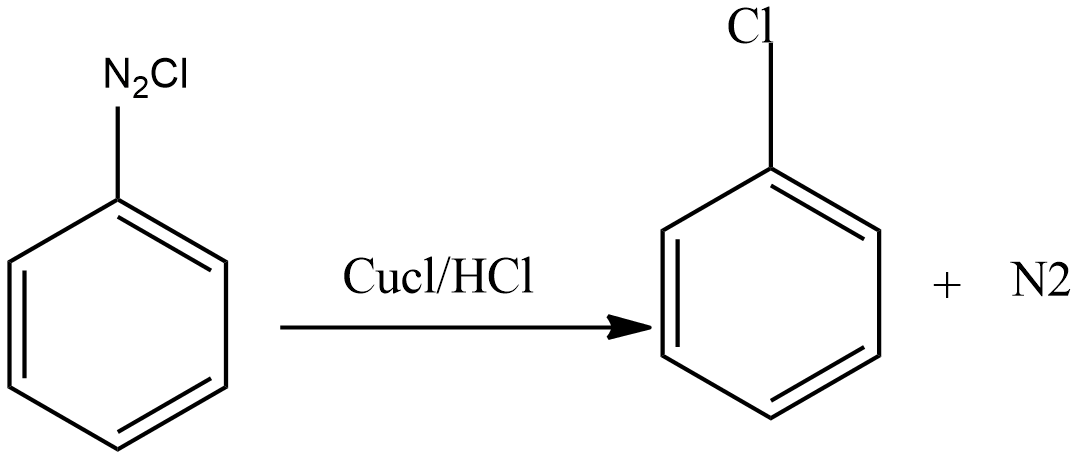
Gattermann reaction: This reaction is the modification of the sandmeyer reaction. In this reaction, a mixture of freshly prepared copper powder in the presence of halogen acid is used instead of cuprous halide dissolved in corresponding halogen acid.
First in this reaction also we make nitrous acid from sodium nitrite.
$NaN{O_2} + HCl(dil) \to HONO + NaCl$
Then again nitrous acid reacts with aniline to form diazonium chloride.
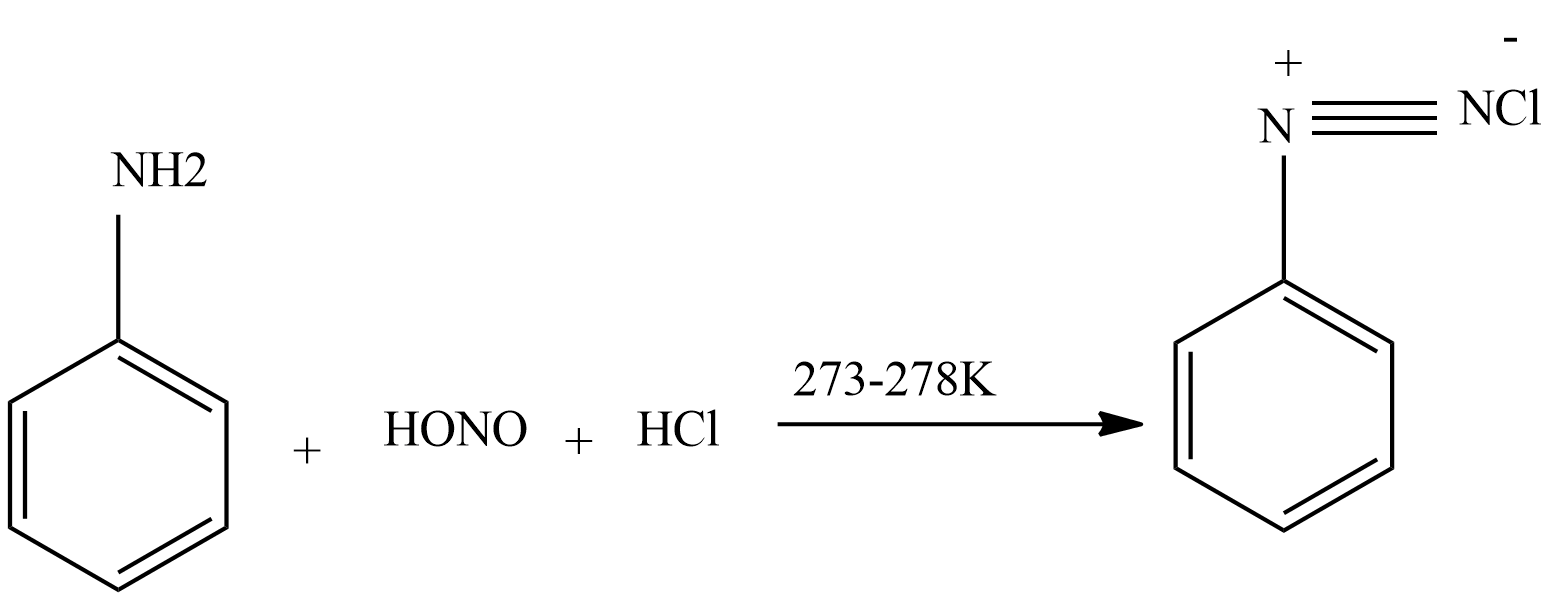
Then diazonium chloride reacts with freshly prepared copper powder in the presence of halogen acid to form aryl halide.
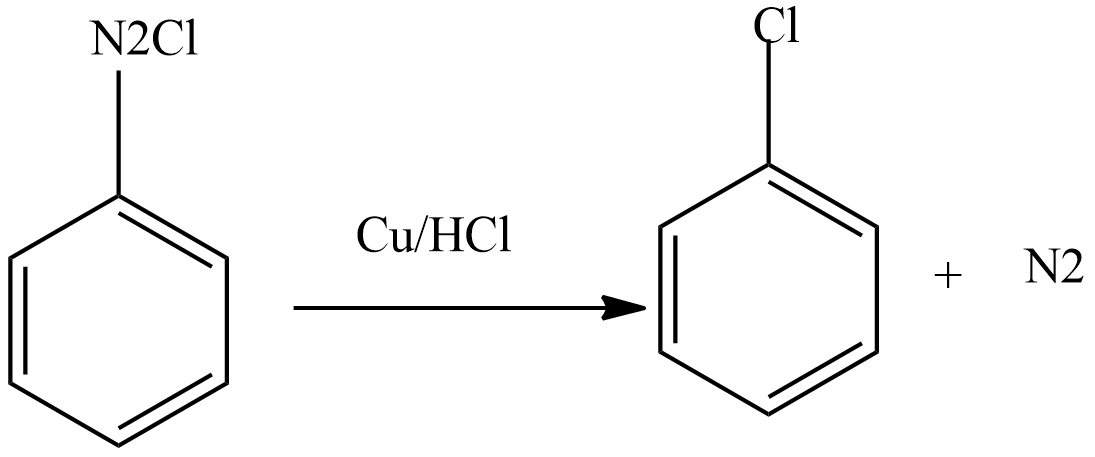
Note:
-Sandmeyer reaction is the two step mechanism reaction. The Sandmeyer reaction follows the free radical mechanism. In this reaction, the halogen attached to copper enters the benzene ring.
-The yield obtained by the Gattermann reaction is 40%.
Complete step by step answer:
Sandmeyer reaction: In this reaction when diazonium salts are treated with $CuCl$ dissolved in $HCl$ or $CuBr$ dissolved in $HBr$ then formation of aryl chloride or aryl bromide takes place.
The sandmeyer reaction is written as:
In the first step we form nitrous acid from sodium nitrite.
$NaN{O_2} + HCl(dil) \to HONO + NaCl$
Then nitrous acid reacts with aniline to form diazonium salt.
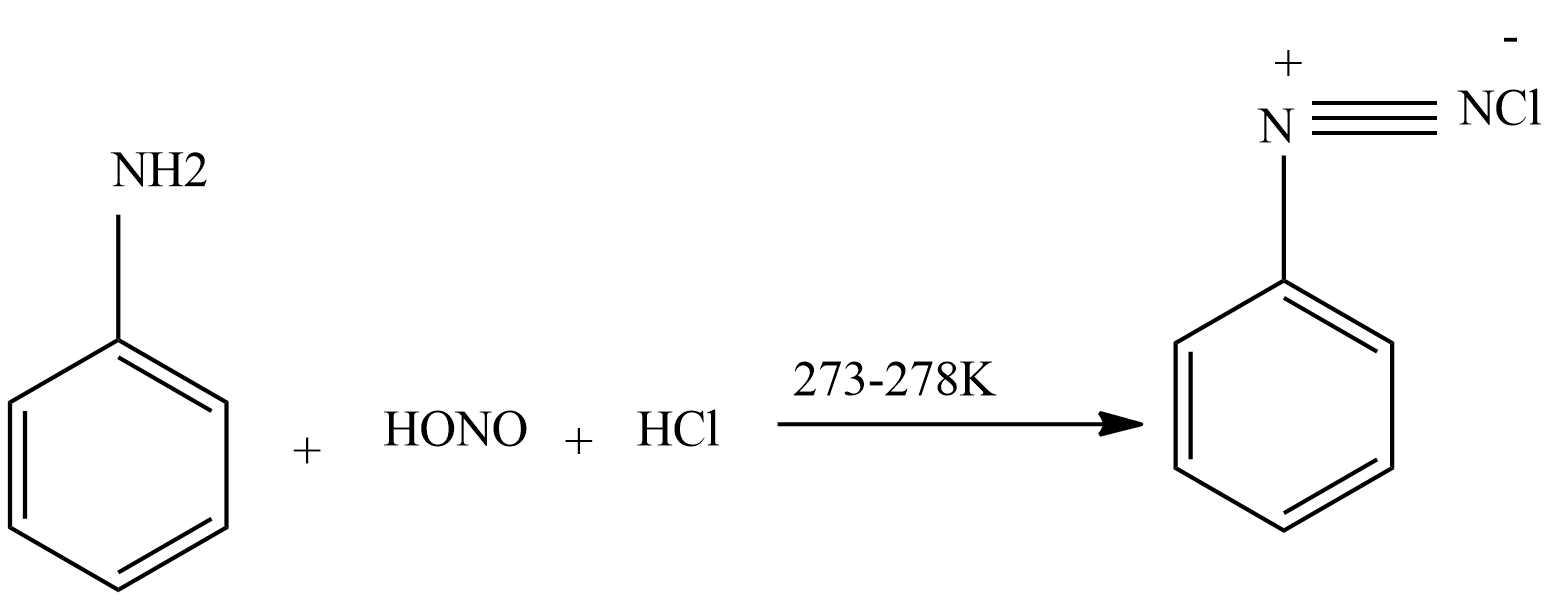
Then diazonium salt reacts with $CuCl$ dissolved in $HCl$ to form chloroarenes.

Gattermann reaction: This reaction is the modification of the sandmeyer reaction. In this reaction, a mixture of freshly prepared copper powder in the presence of halogen acid is used instead of cuprous halide dissolved in corresponding halogen acid.
First in this reaction also we make nitrous acid from sodium nitrite.
$NaN{O_2} + HCl(dil) \to HONO + NaCl$
Then again nitrous acid reacts with aniline to form diazonium chloride.
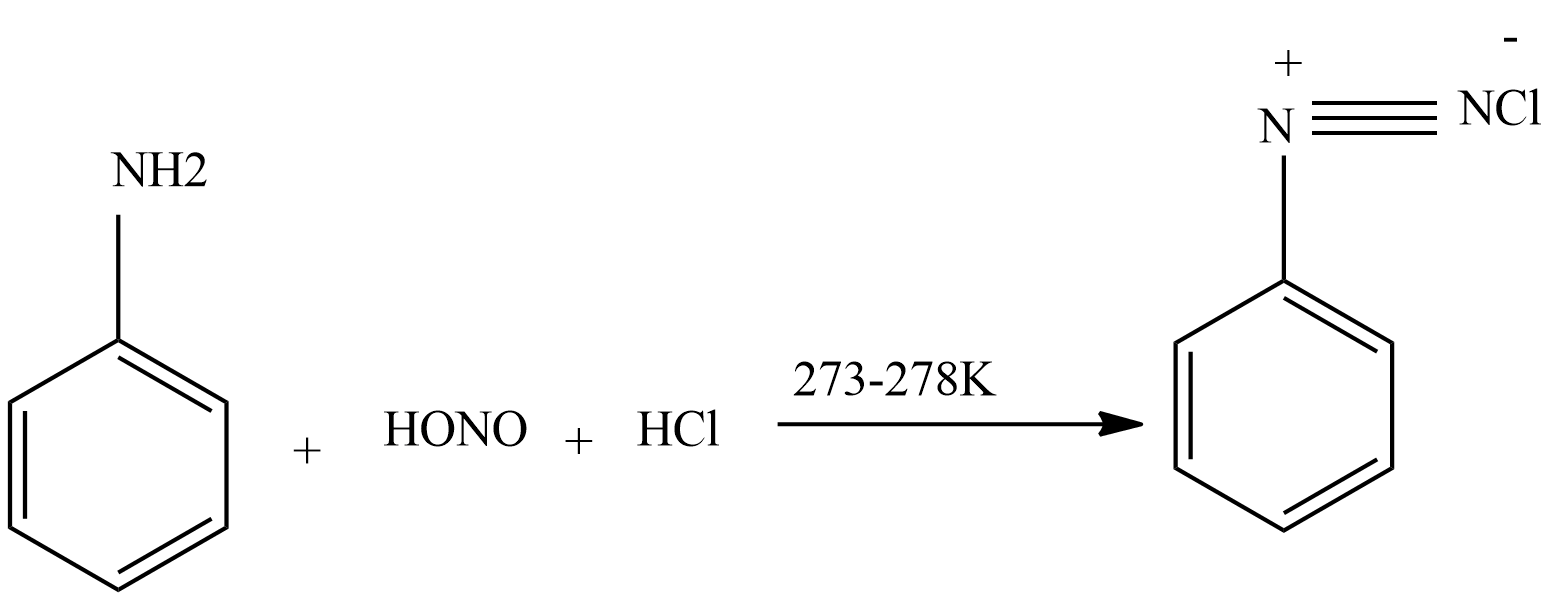
Then diazonium chloride reacts with freshly prepared copper powder in the presence of halogen acid to form aryl halide.

Note:
-Sandmeyer reaction is the two step mechanism reaction. The Sandmeyer reaction follows the free radical mechanism. In this reaction, the halogen attached to copper enters the benzene ring.
-The yield obtained by the Gattermann reaction is 40%.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




