
Explain the following magnetic properties of solids with suitable examples.
(a) Diamagnetic
(b) Paramagnetic
(c) Ferromagnetic
Answer
579.9k+ views
Hint: The magnetic properties of the solids are the results of the magnetic property of the atoms or ions of the solid within them. i.e. the magnetization and magnetism will depend on the movement of electrons within the atom or ion of the solid. According to the magnetic behaviour of the solids the most materials are classified into diamagnetic, paramagnetic and ferromagnetic.
Complete step by step answer:
(a)Diamagnetic: The materials with diamagnetic behaviours are slightly repelled in the magnetic field and those materials do not retain magnetic properties when an external field is removed. They have weak and negative susceptibility to magnetic fields. In diamagnetic materials all the electrons in the atom or ion are in paired condition, so there is no permanent net magnetic moment per atom. Diamagnetic materials contain most elements from periodic tables including copper, silver and gold.

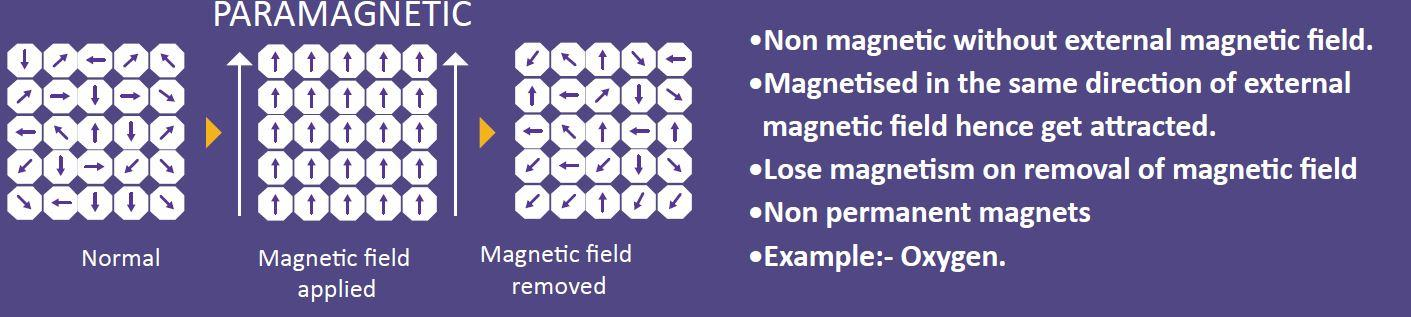
(b)Paramagnetic: These types of materials are slightly attracted by a magnetic field and the material does not retain the magnetic behaviour after the removal of the external magnetic field. Paramagnetic properties are due to the presence of some unpaired electrons. They have small and positive susceptibility to external magnetic fields. Paramagnetic materials contain magnesium, molybdenum, lithium and tantalum.
(c)Ferromagnetic: They possess a strong attraction to magnetic fields and retain the magnetic behaviour after the removal of the external magnetic field. Ferromagnetic substances have some unpaired electrons, so their atoms have net magnetic moment. They have strong magnetic behaviour due the presence of magnetic moments. Iron, cobalt and nickel are the examples of the ferromagnetic material.

Note:
Other than diamagnetic, paramagnetic and ferromagnetic there are two types of materials, i.e. ferromagnetic and antiferromagnetic materials. Ferri-magnetic materials have unequally aligned magnetic moments. Also, weakly attracted to magnetic fields. They will lose their magnetic properties upon heating the substance. Anti-ferromagnetic materials have equally and oppositely aligned domains. So they will cancel each other.
Complete step by step answer:
(a)Diamagnetic: The materials with diamagnetic behaviours are slightly repelled in the magnetic field and those materials do not retain magnetic properties when an external field is removed. They have weak and negative susceptibility to magnetic fields. In diamagnetic materials all the electrons in the atom or ion are in paired condition, so there is no permanent net magnetic moment per atom. Diamagnetic materials contain most elements from periodic tables including copper, silver and gold.

(b)Paramagnetic: These types of materials are slightly attracted by a magnetic field and the material does not retain the magnetic behaviour after the removal of the external magnetic field. Paramagnetic properties are due to the presence of some unpaired electrons. They have small and positive susceptibility to external magnetic fields. Paramagnetic materials contain magnesium, molybdenum, lithium and tantalum.

(c)Ferromagnetic: They possess a strong attraction to magnetic fields and retain the magnetic behaviour after the removal of the external magnetic field. Ferromagnetic substances have some unpaired electrons, so their atoms have net magnetic moment. They have strong magnetic behaviour due the presence of magnetic moments. Iron, cobalt and nickel are the examples of the ferromagnetic material.

Note:
Other than diamagnetic, paramagnetic and ferromagnetic there are two types of materials, i.e. ferromagnetic and antiferromagnetic materials. Ferri-magnetic materials have unequally aligned magnetic moments. Also, weakly attracted to magnetic fields. They will lose their magnetic properties upon heating the substance. Anti-ferromagnetic materials have equally and oppositely aligned domains. So they will cancel each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




