
Explain the following:
Bidentate ligand with an example.
Answer
489.9k+ views
Hint: A ligand is an ion or molecule, which donates a couple of electrons to the vital steel atom or ion to shape a coordination complicated. The word ligand has come from a Latin word, which means “bind”. Ligands may be anions, cations, and impartial molecules. Ligands act as Lewis bases (donate electron pairs) and vital steel atoms regarded as Lewis acid (electron pair acceptor). The nature of bonding among steel to ligand varies from covalent bond to ionic bond.
Complete answer:
Bidentate ligand is a ligand that has “teeth '' or atoms that coordinate without delay to the vital atom in a complex. An instance of a bidentate ligand is ethylenediamine. An unmarried molecule of ethylene diamine can shape bonds to a steel ion. The bonds are shaped among the steel ions and the nitrogen atoms of ethylene diamine.
The shape of ethylenediamine may be proven under this is an instance of bidentate ligand:
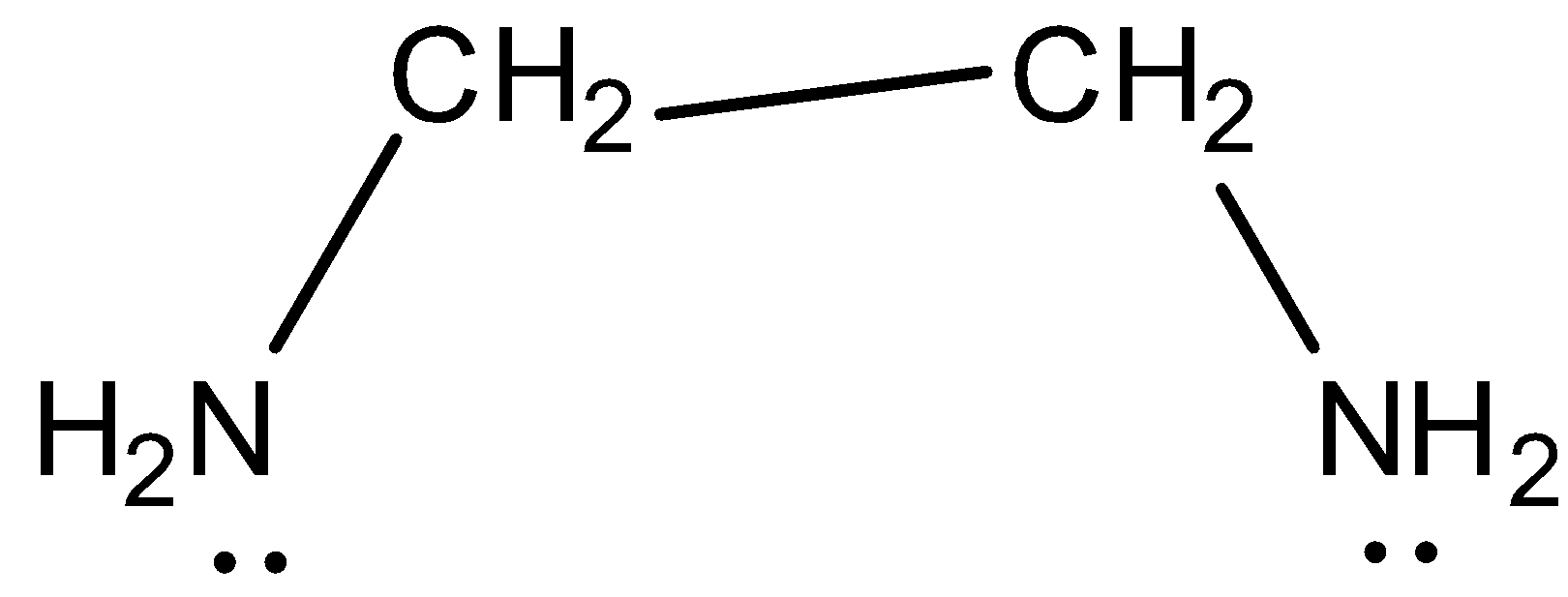
According to coordination chemistry, ligands may be defined as an ion or molecule which can bind to a central metal atom. Ligands are those elements that are negatively charged or neutral in nature. Ligands generally satisfy the primary valency of the coordination complexes. Ligands are regarded as Lewis bases, despite the fact that uncommon instances are recognized to contain Lewis acidic "ligands". Some of the common examples of bidentate ligands are ethylene diamine, oxalate etc.
Note:
Metals and metalloids are certain to ligands in nearly all circumstances, despite the fact that gaseous "naked" steel ions may be generated in an excessive vacuum. Ligands in a complex dictate the reactivity of the vital atom, which includes ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand choice is an important attention in lots of sensible areas, which includes bioorganic and medicinal chemistry, homogeneous catalysis, and environmental chemistry.
Complete answer:
Bidentate ligand is a ligand that has “teeth '' or atoms that coordinate without delay to the vital atom in a complex. An instance of a bidentate ligand is ethylenediamine. An unmarried molecule of ethylene diamine can shape bonds to a steel ion. The bonds are shaped among the steel ions and the nitrogen atoms of ethylene diamine.
The shape of ethylenediamine may be proven under this is an instance of bidentate ligand:

According to coordination chemistry, ligands may be defined as an ion or molecule which can bind to a central metal atom. Ligands are those elements that are negatively charged or neutral in nature. Ligands generally satisfy the primary valency of the coordination complexes. Ligands are regarded as Lewis bases, despite the fact that uncommon instances are recognized to contain Lewis acidic "ligands". Some of the common examples of bidentate ligands are ethylene diamine, oxalate etc.
Note:
Metals and metalloids are certain to ligands in nearly all circumstances, despite the fact that gaseous "naked" steel ions may be generated in an excessive vacuum. Ligands in a complex dictate the reactivity of the vital atom, which includes ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand choice is an important attention in lots of sensible areas, which includes bioorganic and medicinal chemistry, homogeneous catalysis, and environmental chemistry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




