
Explain structural isomerism with suitable examples.
Answer
563.4k+ views
Hint: Isomers are molecules that have the same molecular formula, but have a different structural arrangement of the atoms in space. This is carried out by rearranging the positions of the different atoms present in the compound.
Complete step by step answer:
The different arrangements in structural isomers are possible due to the molecule rotating as a whole or rotating about particular bonds.
In structural isomerism, the order of arrangement of atoms are different. This can be understood easily with the help of some examples. Alkanes are the simplest class of organic compounds that show structural isomerism. They contain only tetravalent (making 4 covalent bonds) carbon atoms and hydrogen who can arrange themselves in different orientations. Butane, methylpropane etc. are examples for this.
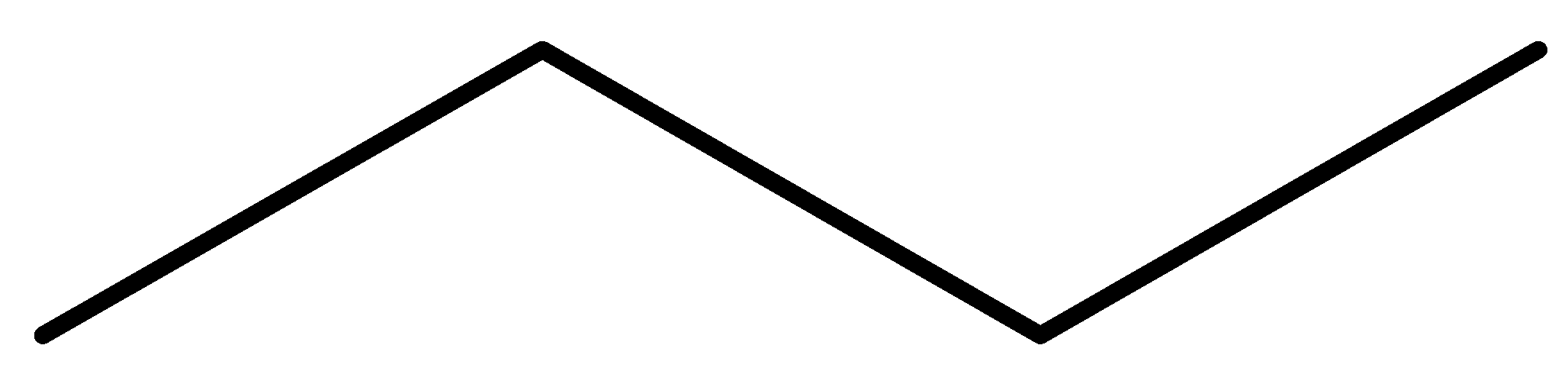 n-Butane
n-Butane
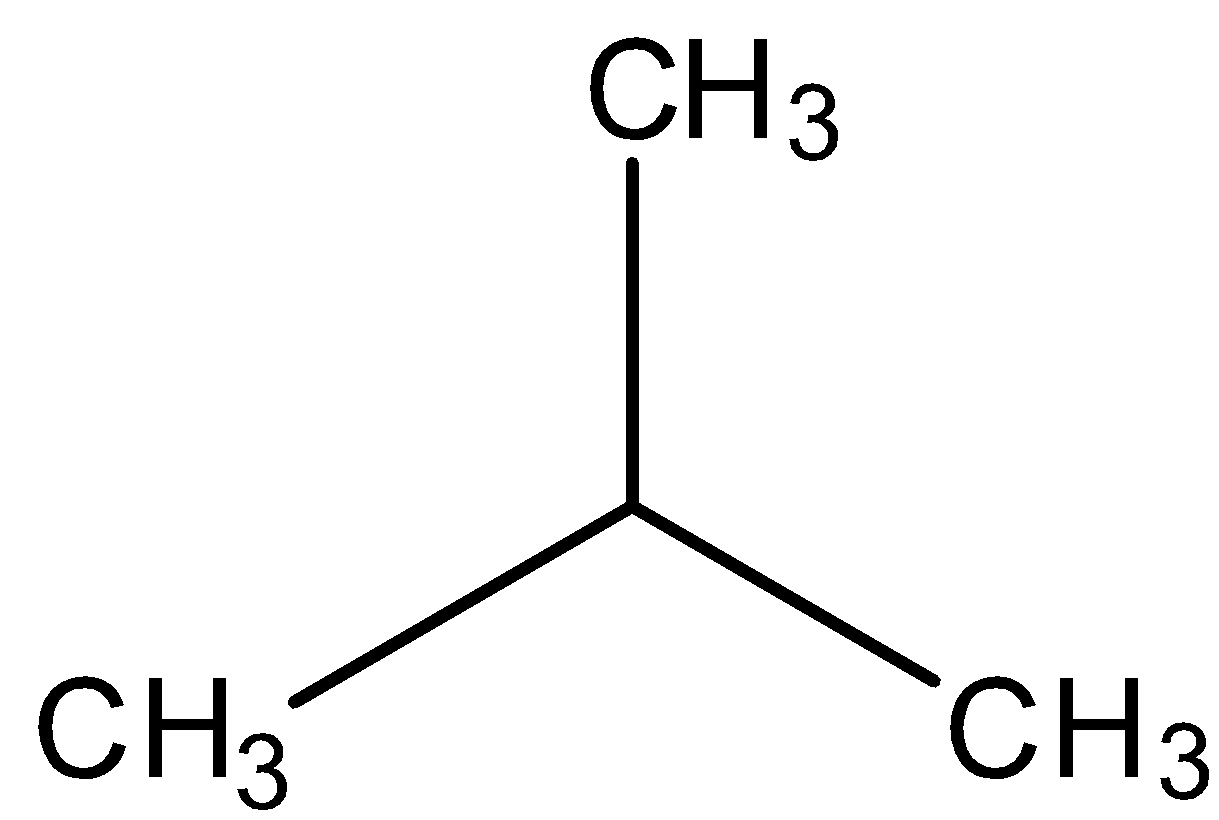 methylpropane
methylpropane
We can see that in both of these structures, there are only carbon and hydrogen atoms and they can arrange themselves to different orientations in space.
There are different types of structural isomerism and they are namely chain isomerism, position isomerism and functional group isomerism. All these are exhibited by compounds having atoms of the same kind. In position isomerism, the positions of the atoms in the compound change and in functional group isomerism also the position of the various functional groups substituted in the chain change and we obtain functional group isomers. In chain isomerism, the orientation of the chain differs in each of the isomers as the position of atoms differ in the chain.
Additional Information:
There are various types of isomerism and the two main forms are structural isomerism or constitutional isomerism and the main other type is spatial or stereo isomerism. In both of these, the molecular formulas are the same.
Note: Isomers are compounds that have the same molecular formula but different structural orientations in the world. These compounds are of a variety of uses in the industry because when they orient in different arrangements, their properties may also differ.
Complete step by step answer:
The different arrangements in structural isomers are possible due to the molecule rotating as a whole or rotating about particular bonds.
In structural isomerism, the order of arrangement of atoms are different. This can be understood easily with the help of some examples. Alkanes are the simplest class of organic compounds that show structural isomerism. They contain only tetravalent (making 4 covalent bonds) carbon atoms and hydrogen who can arrange themselves in different orientations. Butane, methylpropane etc. are examples for this.


We can see that in both of these structures, there are only carbon and hydrogen atoms and they can arrange themselves to different orientations in space.
There are different types of structural isomerism and they are namely chain isomerism, position isomerism and functional group isomerism. All these are exhibited by compounds having atoms of the same kind. In position isomerism, the positions of the atoms in the compound change and in functional group isomerism also the position of the various functional groups substituted in the chain change and we obtain functional group isomers. In chain isomerism, the orientation of the chain differs in each of the isomers as the position of atoms differ in the chain.
Additional Information:
There are various types of isomerism and the two main forms are structural isomerism or constitutional isomerism and the main other type is spatial or stereo isomerism. In both of these, the molecular formulas are the same.
Note: Isomers are compounds that have the same molecular formula but different structural orientations in the world. These compounds are of a variety of uses in the industry because when they orient in different arrangements, their properties may also differ.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




