
Explain Markovnikov’s and Anti-Markovnikov’s rule using reaction mechanism.
Answer
585k+ views
Hint: Addition of HBr to propene (unsymmetrical alkene) in the absence of peroxide results in the formation of 2-bromopropane as major product by Markoviknov’s rule while in the presence of peroxide results in the formation of 1-bromopropane as major product by Anti-markovnikov’s rule. Markovnikov’s rule mechanism depends upon the stability of carbocation intermediate and Anti-Markovnikov’s rule mechanism depends upon the stability of free radical intermediate.
Complete step by step answer:
The addition of HX (hydrogen halides) to an unsymmetrical alkene is a regioselective reaction. In case of an unsymmetrical alkene like propene, reaction with HBr may result in the formation of two products, namely 1-bromopropane and 2-bromopropane. However, only one is more selective. Markovnikov, a Russian chemist made a generalisation in 1869 after studying such reactions in detail. Markovnikov framed a rule called Markovnikov’s rule which states that the negative part of the addendum (polar reagent) gets attached to that carbon atom which possesses a lesser number of hydrogen atoms. This generation of Markovnikov’s rule can be better understood in terms of mechanism of a reaction.
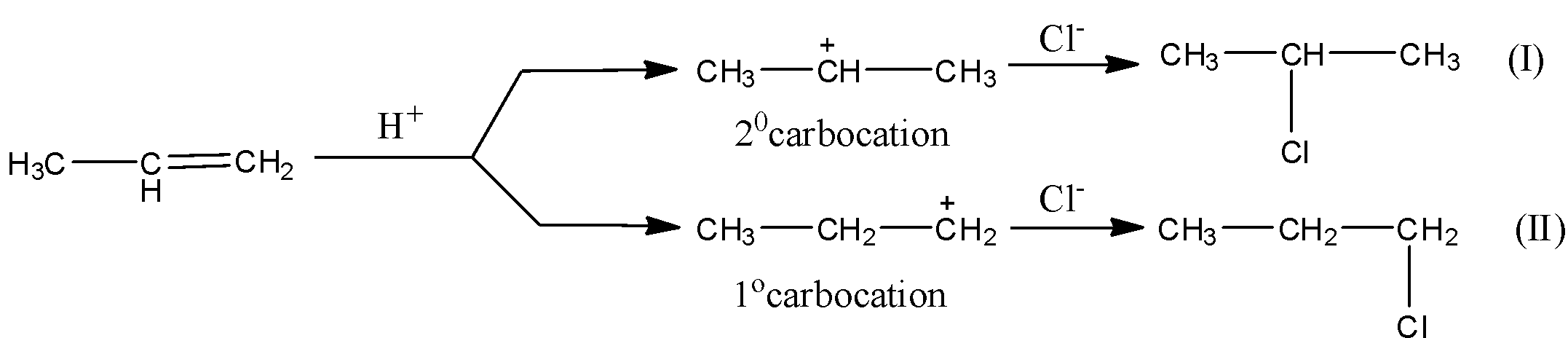
Mechanism to Markovnikov’s rule: Let us consider the mechanism of addition of HCl to propene. Hydrogen chloride provides an electrophile, ${H^ + }$, which attacks the double bond of propene. The attack of electrophile on either of the olefinic carbons may result in the formation of a ${1^{\text{o}}}$ or a ${2^{\text{o}}}$ carbocation. We know that ${2^{\text{o}}}$ carbocation is always more stable than ${1^{\text{o}}}$ carbocation, therefore, the product (I), that is, 2-chloropropane forms predominantly because ${2^{\text{o}}}$ carbocation formed at a faster rate.
Anti-Markovnikov’s rule: In the presence of peroxide, addition of HBr to unsymmetrical alkenes like propene takes place contrary to the Markovnikov’s rule. This additional reaction was observed by M.S. Kharash and F.R. Mayo in 1933. This reaction is also known as peroxide effect or kharash effect or Anti-Markovnikov addition.
Mechanism to Anti-Markovnikov’s rule: Peroxide effect or Anti-Markovnikov addition proceeds via free radical mechanism as given below:
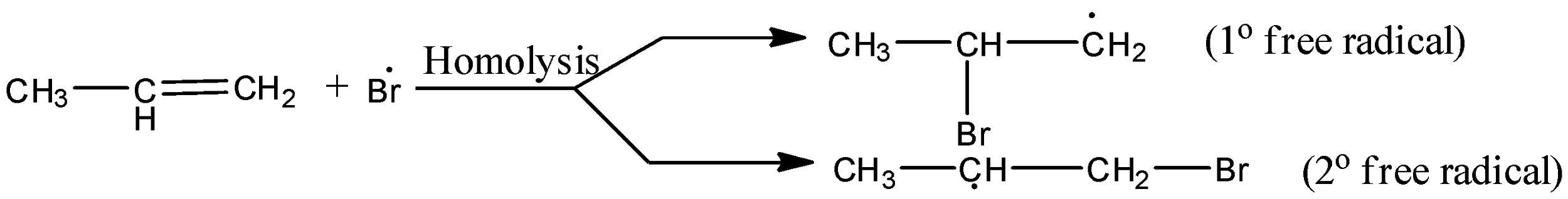
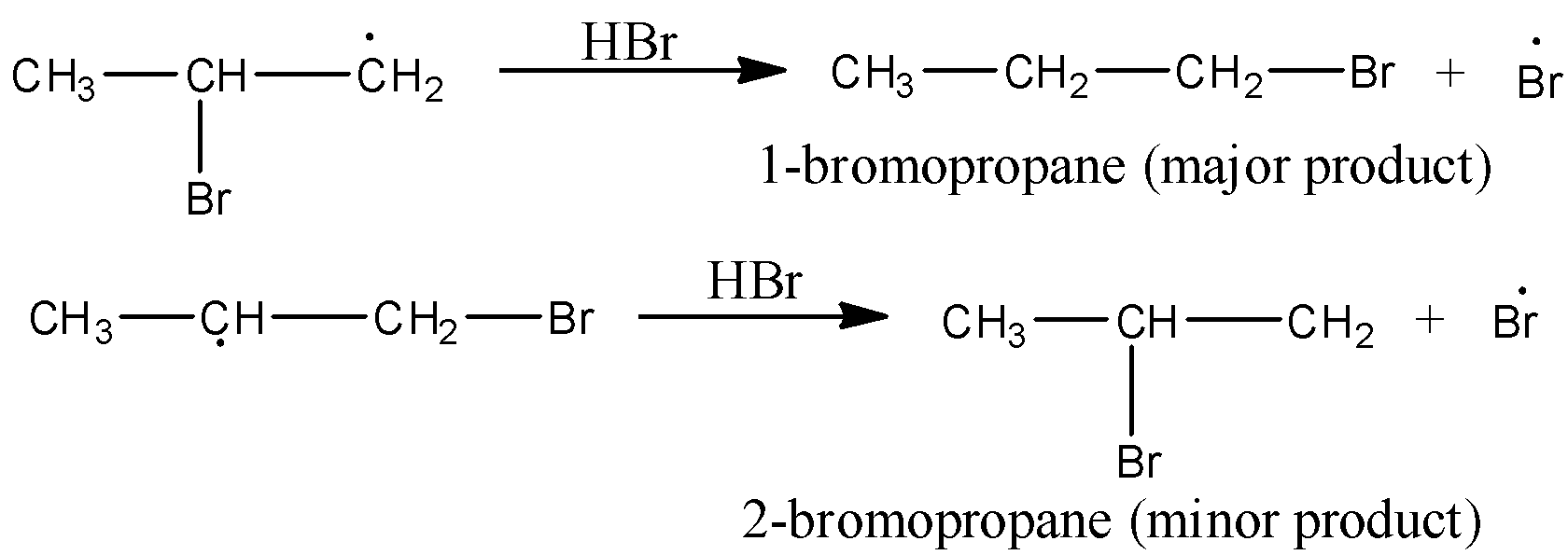
The secondary (${2^{\text{o}}}$) free radical obtained in the mechanism is more stable than the primary (${1^{\text{o}}}$) free radical. This explains the formation of 1-bromopropane as the major product.
Note: It may be noted that the peroxide effect is not observed in addition to HCl and HI. This may be due to the reason that the H-Cl bond is stronger than H-Br bond, so it is not cleaved by the free radicals, whereas the H-I bond is quite weaker and iodine free radicals combine to form iodine molecules instead of adding to the double bond of olefinic carbons.
Complete step by step answer:
The addition of HX (hydrogen halides) to an unsymmetrical alkene is a regioselective reaction. In case of an unsymmetrical alkene like propene, reaction with HBr may result in the formation of two products, namely 1-bromopropane and 2-bromopropane. However, only one is more selective. Markovnikov, a Russian chemist made a generalisation in 1869 after studying such reactions in detail. Markovnikov framed a rule called Markovnikov’s rule which states that the negative part of the addendum (polar reagent) gets attached to that carbon atom which possesses a lesser number of hydrogen atoms. This generation of Markovnikov’s rule can be better understood in terms of mechanism of a reaction.
Mechanism to Markovnikov’s rule: Let us consider the mechanism of addition of HCl to propene. Hydrogen chloride provides an electrophile, ${H^ + }$, which attacks the double bond of propene. The attack of electrophile on either of the olefinic carbons may result in the formation of a ${1^{\text{o}}}$ or a ${2^{\text{o}}}$ carbocation. We know that ${2^{\text{o}}}$ carbocation is always more stable than ${1^{\text{o}}}$ carbocation, therefore, the product (I), that is, 2-chloropropane forms predominantly because ${2^{\text{o}}}$ carbocation formed at a faster rate.

Anti-Markovnikov’s rule: In the presence of peroxide, addition of HBr to unsymmetrical alkenes like propene takes place contrary to the Markovnikov’s rule. This additional reaction was observed by M.S. Kharash and F.R. Mayo in 1933. This reaction is also known as peroxide effect or kharash effect or Anti-Markovnikov addition.
Mechanism to Anti-Markovnikov’s rule: Peroxide effect or Anti-Markovnikov addition proceeds via free radical mechanism as given below:

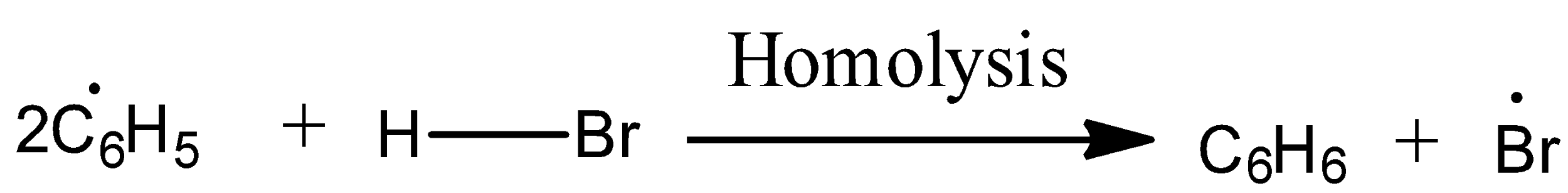


The secondary (${2^{\text{o}}}$) free radical obtained in the mechanism is more stable than the primary (${1^{\text{o}}}$) free radical. This explains the formation of 1-bromopropane as the major product.
Note: It may be noted that the peroxide effect is not observed in addition to HCl and HI. This may be due to the reason that the H-Cl bond is stronger than H-Br bond, so it is not cleaved by the free radicals, whereas the H-I bond is quite weaker and iodine free radicals combine to form iodine molecules instead of adding to the double bond of olefinic carbons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




