
Example of a three - dimensional silicate is:
A. Zeolites
B. Ultramarines
C. feldspars
D. beryls
Answer
587.7k+ views
Hint: For this problem, we have to study the three-dimensional structure of the given molecules. As the 3-D structure will have the most complex structure and consist of silicon, so the molecule which has silicon in the complex structure will be the correct answer.
Complete step by step answer:
- In the given question, we have to explain the correct examples of three - dimensional silicates.
- As we know that the minerals which consist of silica and oxygen in the tetrahedral form that is $\text{SiO}_{4}^{-4}$ unit is called the silica mineral.
- There are many forms of silicate ions such as sheet silicates, double-chain silicate, chain silicates, cyclic silicates, three - dimensional silicates, etc.
- In three - dimensional silicates, the oxygen atoms that are present in the silicates are shared with the adjacent tetrahedron.
- The examples of the three - dimensional silicates are zeolites ultramarines, feldspar, etc which are found in the crystalline form.
- So, we can say that the molecules in option A, B and C are the examples of three - dimensional silicates.
- Moreover, when the silica is replaced by the aluminium atom then the structure is known as aluminosilicates which are also three - dimensional.
- Whereas the beryl is an example of cyclic silicate because three silicate structures make a ring-like structure or cyclic structure.
- The molecular formula of the beryl is $\text{B}{{\text{e}}_{3}}\text{A}{{\text{l}}_{2}}{{(\text{Si}{{\text{O}}_{3}})}_{6}}$ whereas the molecular formula of feldspar is $\text{KAlS}{{\text{i}}_{3}}{{\text{O}}_{8}}$ and the molecular formula of ultramarines will be $\text{N}{{\text{a}}_{8}}{{(\text{SiAl}{{\text{O}}_{4}})}_{6}}\text{.(}{{\text{S}}_{3}}{{)}_{6}}$.
So, the correct answer is “Option A, B and C”.
Note: The structure of the zeolite is complex and 3D dimensional as shown below:
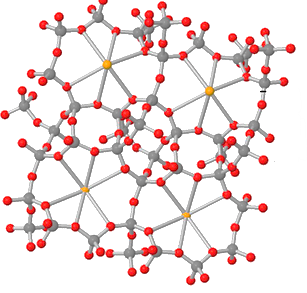
here the grey coloured circles are silicon or aluminium and red coloured balls are oxygen atoms.
Complete step by step answer:
- In the given question, we have to explain the correct examples of three - dimensional silicates.
- As we know that the minerals which consist of silica and oxygen in the tetrahedral form that is $\text{SiO}_{4}^{-4}$ unit is called the silica mineral.
- There are many forms of silicate ions such as sheet silicates, double-chain silicate, chain silicates, cyclic silicates, three - dimensional silicates, etc.
- In three - dimensional silicates, the oxygen atoms that are present in the silicates are shared with the adjacent tetrahedron.
- The examples of the three - dimensional silicates are zeolites ultramarines, feldspar, etc which are found in the crystalline form.
- So, we can say that the molecules in option A, B and C are the examples of three - dimensional silicates.
- Moreover, when the silica is replaced by the aluminium atom then the structure is known as aluminosilicates which are also three - dimensional.
- Whereas the beryl is an example of cyclic silicate because three silicate structures make a ring-like structure or cyclic structure.
- The molecular formula of the beryl is $\text{B}{{\text{e}}_{3}}\text{A}{{\text{l}}_{2}}{{(\text{Si}{{\text{O}}_{3}})}_{6}}$ whereas the molecular formula of feldspar is $\text{KAlS}{{\text{i}}_{3}}{{\text{O}}_{8}}$ and the molecular formula of ultramarines will be $\text{N}{{\text{a}}_{8}}{{(\text{SiAl}{{\text{O}}_{4}})}_{6}}\text{.(}{{\text{S}}_{3}}{{)}_{6}}$.
So, the correct answer is “Option A, B and C”.
Note: The structure of the zeolite is complex and 3D dimensional as shown below:

here the grey coloured circles are silicon or aluminium and red coloured balls are oxygen atoms.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




