
Ethylidene chloride is a/an
A. vicinal-dihalide
B. Gem-halide
C. allylic halide
D. Vinylic halide
Answer
590.1k+ views
Hint: Ethylidene chloride is a Geminal Dihalide. The compound that has two halogen atoms on the same carbon atom are known as geminal dihalides. Vicinal dihalide means the presence of two chlorine atoms on two different and adjacent carbon atoms.
Complete step by step answer:
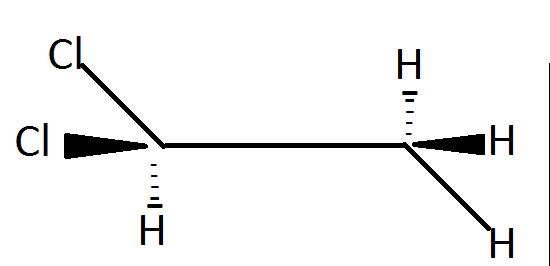
Vicinal dihalides, compounds that have halogens on adjacent carbons, are prepared by the reaction between a halogen and an alkene. The simplest example is the reaction between ethylene and chlorine to give \[1,2 - dichloroethane\] (ethylene dichloride). Therefore the given compound is not a Vicinal dihalide.
Allylic halides are the compounds in which the halogen atom is bonded to an \[s{p^3}\] - hybridized carbon atom next to the carbon - carbon double bond (\[C = C\]). Hence the given compound is not an allylic halide.
Vinyl halide is a Hydrocarbon in which the halogen (\[X = {\text{ }}Cl,{\text{ }}I,{\text{ }}F,{\text{ }}Br\]) is directly attached to an alpha carbon (\[s{p^2}\] hybridised carbon atom).
Gem dihalides have halogen atoms attached to the same carbon atom and in the given compound both of the chlorine atoms are attached to the same carbon atom.
Hence, the correct option is (B), Gem-halide .
Note:
Students have confusion in geminal and vicinal dihalides. Make sure to confirm that Gem dihalides have halogens attached to the same carbon atom while Vicinal dihalides are the compounds that have halogens on adjacent carbon atoms, and vinylic halide means halogen is directly connected with a double bonded carbon atom.
Complete step by step answer:

Vicinal dihalides, compounds that have halogens on adjacent carbons, are prepared by the reaction between a halogen and an alkene. The simplest example is the reaction between ethylene and chlorine to give \[1,2 - dichloroethane\] (ethylene dichloride). Therefore the given compound is not a Vicinal dihalide.
Allylic halides are the compounds in which the halogen atom is bonded to an \[s{p^3}\] - hybridized carbon atom next to the carbon - carbon double bond (\[C = C\]). Hence the given compound is not an allylic halide.
Vinyl halide is a Hydrocarbon in which the halogen (\[X = {\text{ }}Cl,{\text{ }}I,{\text{ }}F,{\text{ }}Br\]) is directly attached to an alpha carbon (\[s{p^2}\] hybridised carbon atom).
Gem dihalides have halogen atoms attached to the same carbon atom and in the given compound both of the chlorine atoms are attached to the same carbon atom.
Hence, the correct option is (B), Gem-halide .
Note:
Students have confusion in geminal and vicinal dihalides. Make sure to confirm that Gem dihalides have halogens attached to the same carbon atom while Vicinal dihalides are the compounds that have halogens on adjacent carbon atoms, and vinylic halide means halogen is directly connected with a double bonded carbon atom.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




