
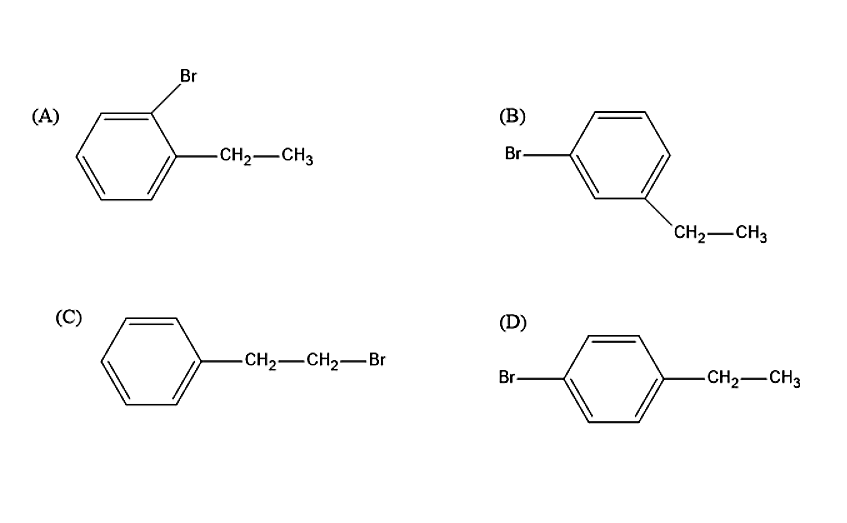
Ethylbenzene with bromine in presence of \[FeB{r_3}\] predominantly gives:

Answer
576k+ views
Hint: \[FeB{r_3}\] is a strong Lewis acid. It will catalyse the Bromination reaction of Ethylbenzene. This reaction can give three products such as ortho isomer and para isomer. From these isomers we have to find which of the isomers is the major product.
Complete Solution :
Let us see now what product will be produced when ethylbenzene reacts with the Bromine molecule in presence of \[FeB{r_3}\]. Bromine is not a strong electrophile to react with ethylbenzene. Therefore, a strong Lewis acid such as \[FeB{r_3}\] is added, in order to catalyse the reaction. Thus, leads to the substitution product. The Bromine molecule will donate its electron pair to \[FeB{r_3}\], to create more polar Br-Br bond and thus, a strong electrophile is formed. The ethyl group in ethylbenzene is an ortho-para directing group. Thus, the product formed in the reaction will be ortho-BromoEthylbenzene and para-BromoEthylbenzene.
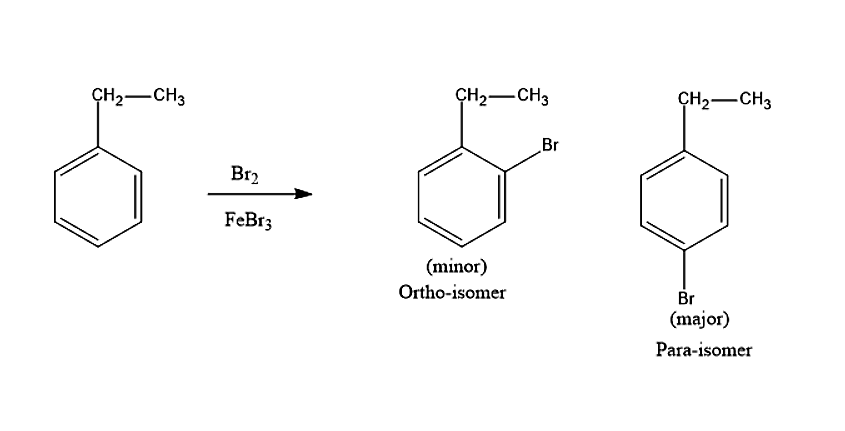
The steric hindrance is less in para isomer therefore, it is the major product.
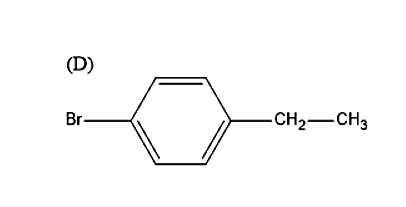
So, the correct answer is “Option D”.
Note: The presence of the alkyl group in benzene will make the benzene undergo EAS reaction faster than the benzene group. This will give the product of ortho and para isomers, as the alkyl group is an ortho-para directing group and is an activating group.
Complete Solution :
Let us see now what product will be produced when ethylbenzene reacts with the Bromine molecule in presence of \[FeB{r_3}\]. Bromine is not a strong electrophile to react with ethylbenzene. Therefore, a strong Lewis acid such as \[FeB{r_3}\] is added, in order to catalyse the reaction. Thus, leads to the substitution product. The Bromine molecule will donate its electron pair to \[FeB{r_3}\], to create more polar Br-Br bond and thus, a strong electrophile is formed. The ethyl group in ethylbenzene is an ortho-para directing group. Thus, the product formed in the reaction will be ortho-BromoEthylbenzene and para-BromoEthylbenzene.

The steric hindrance is less in para isomer therefore, it is the major product.

So, the correct answer is “Option D”.
Note: The presence of the alkyl group in benzene will make the benzene undergo EAS reaction faster than the benzene group. This will give the product of ortho and para isomers, as the alkyl group is an ortho-para directing group and is an activating group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




