
Ethane, with the molecular formula ${{\text{C}}_{2}}{{\text{H}}_{6}}$ has:
A. 6 covalent bonds
B. 7 covalent bonds
C. 8 covalent bonds
D. 9 covalent bonds
Answer
522.3k+ views
Hint: Alkanes are the saturated hydrocarbon which is also known as paraffin. A covalent bond is a bond in which the sharing of a pair of electrons takes place between two atoms. An atom having more electronegativity can attract the pair of electrons more easily.
Complete answer:
Alkanes form an only single bond between different atoms.
So, to calculate the number of covalent bonds we have to draw the structure of ethane:
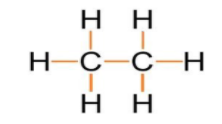
As we can see that one covalent bond is present between two carbon atoms and six covalent bonds are present between hydrogen and carbon atoms which are highlighted with the orange colour.
So, option B. is the correct answer.
Additional Information Ethane is an organic compound which is used for the synthesis of ethylene.
- It is an odourless and colourless compound with a common name of dimethyl.
- Ethane is also soluble in water and can be used to make ethanol.
- The hybridisation of the ethane molecule will be \[\text{s}{{\text{p}}^{3}}\] with a geometry of trigonal planar.
- Here, in this molecule, the carbon and hydrogen have less electronegativity difference whereas the carbon-carbon bond has no electronegativity difference, so the electrons remain at the centre of the bond.
Note: Carbons cannot gain as well as a loss any electron so to fulfil their octet they can only share a pair of electrons to become stable. The compound which consists of covalent bonds usually has a low melting point.
Complete answer:
Alkanes form an only single bond between different atoms.
So, to calculate the number of covalent bonds we have to draw the structure of ethane:

As we can see that one covalent bond is present between two carbon atoms and six covalent bonds are present between hydrogen and carbon atoms which are highlighted with the orange colour.
So, option B. is the correct answer.
Additional Information Ethane is an organic compound which is used for the synthesis of ethylene.
- It is an odourless and colourless compound with a common name of dimethyl.
- Ethane is also soluble in water and can be used to make ethanol.
- The hybridisation of the ethane molecule will be \[\text{s}{{\text{p}}^{3}}\] with a geometry of trigonal planar.
- Here, in this molecule, the carbon and hydrogen have less electronegativity difference whereas the carbon-carbon bond has no electronegativity difference, so the electrons remain at the centre of the bond.
Note: Carbons cannot gain as well as a loss any electron so to fulfil their octet they can only share a pair of electrons to become stable. The compound which consists of covalent bonds usually has a low melting point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




