
Electrophilic addition reaction proceeds in two steps. The first step involves the addition of an electrophile. The type of intermediate formed in the first step of the following addition reaction is:
${{\text{H}}_{3}}\text{C - HC = C}{{\text{H}}_{2}}\text{ + }\overset{+}{\mathop{\text{H}}}\,\text{ }\to $
A. $\text{2}{}^\circ $ carbanion
B. $1{}^\circ $ carbocation
C. $\text{2}{}^\circ $ carbocation
D. $1{}^\circ $ carbanion
Answer
591k+ views
Hint: The stability of the carbocation is important because more is the stability of a carbocation more are the chances of the feasibility of the reaction. Carbocation consists of a positive on the carbon atom which is formed by the loss of an electron.
Complete step by step answer:
-In the given question, propene is given to us which is reacting with the hydrogen ion and we have to tell the intermediate product which will be formed.
-As we know that hydrogen ion is an electrophile because it tends to accept a pair of electrons and also consist of a positive charge.
-So, firstly the pi- bond present between central carbon and adjacent carbon breaks and two conditions are formed:
I)
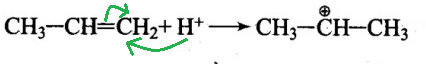
-So, here the hydrogen atom attaches to the carbon which has an extra pair of electrons and the central gains a positive charge which is now known as carbocation or secondary carbocation.
-It is a secondary carbocation because it is connected to two carbon atoms.
II)
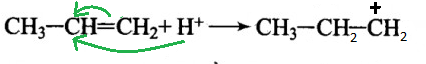
-Here, the carbocation formed is primary because it is connected to only one carbon atom.
-But as we know that primary carbocation is less stable than the secondary carbocation because more is the alkyl group attaches more will the electron density on the carbocation.
-This is so because the alkyl group is the electron-donating group and gives electron density to the neighbouring carbon.
-Hence, the intermediate formed will be the secondary carbocation.
So, the correct answer is “Option C”.
Note: As the name tells electrophilic addition reactions are those in which the electrophile attacks on the unsaturated bond. That’s why a compound must have a double or triple bond to undergo electrophilic addition reaction.
Complete step by step answer:
-In the given question, propene is given to us which is reacting with the hydrogen ion and we have to tell the intermediate product which will be formed.
-As we know that hydrogen ion is an electrophile because it tends to accept a pair of electrons and also consist of a positive charge.
-So, firstly the pi- bond present between central carbon and adjacent carbon breaks and two conditions are formed:
I)
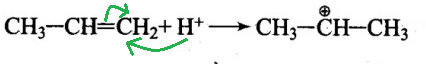
-So, here the hydrogen atom attaches to the carbon which has an extra pair of electrons and the central gains a positive charge which is now known as carbocation or secondary carbocation.
-It is a secondary carbocation because it is connected to two carbon atoms.
II)
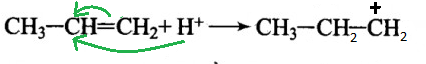
-Here, the carbocation formed is primary because it is connected to only one carbon atom.
-But as we know that primary carbocation is less stable than the secondary carbocation because more is the alkyl group attaches more will the electron density on the carbocation.
-This is so because the alkyl group is the electron-donating group and gives electron density to the neighbouring carbon.
-Hence, the intermediate formed will be the secondary carbocation.
So, the correct answer is “Option C”.
Note: As the name tells electrophilic addition reactions are those in which the electrophile attacks on the unsaturated bond. That’s why a compound must have a double or triple bond to undergo electrophilic addition reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




