
How many electrons are present in the M-shell of an element with atomic number 20?
A) 8
B) 6
C) 18
D) 2
Answer
520k+ views
Hint: K-shell is the first energy level and can have a maximum of 2 electrons. L-shell is the Second energy level and can have a maximum of 8 electrons. M-shell is the third energy level and can have a maximum of 18 electrons.
Complete step by step answer:
In an atom, the electrons surround the nucleus in different orbitals or energy levels. These orbitals are known as either first, second, third ... energy levels or K,L,M, N... shells. K-shell is the first energy level and can have a maximum of 2 electrons. L-shell is the second energy level and can have a maximum of 8 electrons. M-shell is the third energy level and can have a maximum of 8 electrons.
The atomic number of an element is 20. Atomic number is equal to the number of electrons present in a neutral atom. Thus, the element with atomic number 20 has 20 electrons. Out of 20 electrons, 2 electrons are present in the K-shell and 8 electrons are present in the L-shell. Out of the remaining 20-(2+8)=10 electrons, 8 electrons are present in the M-shell and 2 electrons are present in the N shell.
Hence, 8 electrons are present in the M-shell of an element with atomic number 20.
The structure of atom is as shown below:
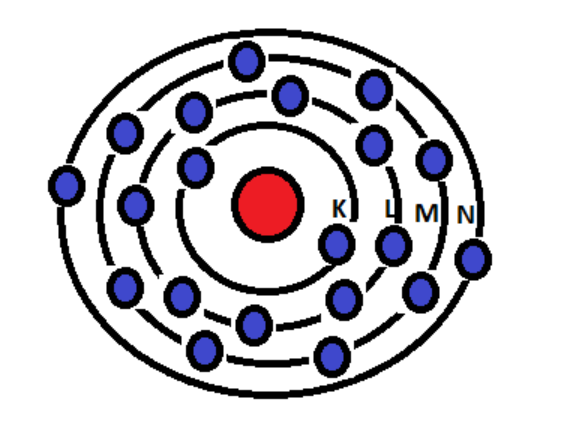
Thus, option A is the correct answer.
Additional information: The electronic configuration of the element with atomic number 20 is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}\].
Note:
Do not put more than 2 electrons in K-shell and more than 8 electrons each in L and M-shells.
Complete step by step answer:
In an atom, the electrons surround the nucleus in different orbitals or energy levels. These orbitals are known as either first, second, third ... energy levels or K,L,M, N... shells. K-shell is the first energy level and can have a maximum of 2 electrons. L-shell is the second energy level and can have a maximum of 8 electrons. M-shell is the third energy level and can have a maximum of 8 electrons.
The atomic number of an element is 20. Atomic number is equal to the number of electrons present in a neutral atom. Thus, the element with atomic number 20 has 20 electrons. Out of 20 electrons, 2 electrons are present in the K-shell and 8 electrons are present in the L-shell. Out of the remaining 20-(2+8)=10 electrons, 8 electrons are present in the M-shell and 2 electrons are present in the N shell.
Hence, 8 electrons are present in the M-shell of an element with atomic number 20.
The structure of atom is as shown below:

Thus, option A is the correct answer.
Additional information: The electronic configuration of the element with atomic number 20 is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}\].
Note:
Do not put more than 2 electrons in K-shell and more than 8 electrons each in L and M-shells.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




