
How many electron pairs are present in valence shell oxygen in water molecules?
\[
A.\;\;\;\;\;4 \\
B.\;\;\;\;\;1 \\
C.\;\;\;\;\;2 \\
D.\;\;\;\;\;3 \\
\]
Answer
522.3k+ views
Hint: Water is a polar molecule. Water has hydrogens and oxygen atoms covalently bonded to each other. Look into the structure of water molecules carefully. Oxygen atom contains two unshared pairs of electrons and with other electrons it forms a bond with the hydrogen atoms.
Complete answer: Water molecules consist of hydrogen and oxygen bonded together with each other. Water exists in all the three states gaseous, liquid and solid. It is an essential component for life.
Water molecule is polar. This means that there is an unequal distribution of electron density. It has partial positive charges near the hydrogen atoms and partial negative charge near the oxygen atoms due to the unshared electron pairs. Presence of such charges on each of these atoms gives a water molecule a net dipole moment. Water molecules are difficult to separate. This is because the electrical attraction between water molecules caused by this dipole pulls the individual atoms hydrogen and oxygen close together, making it more difficult to separate them all.
The structure of molecules is very simple. Each hydrogen and oxygen molecule are bonded to each other with covalent bonds. The structure of water molecule is shown below:
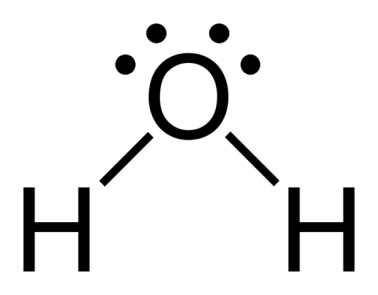
Oxygen has two pair electrons which are not shared. These are lone pairs. Thus, there are$4$electrons surrounding the oxygen atom, two pairs are involved in covalent bonds with the hydrogen atom and two unshared pairs on the opposite side of the oxygen atom.
Therefore the correct option is \[A.\;\;\;\;\;4\].
Note:
Valence shell is the outermost shell of every element. They take part in bonding with the other atoms. Valence shell holds up a fixed number of electrons. The electrons in the oxygen atom which are not being used up for bonding are known as lone pairs. There is covalent bond formation in water molecules as there is not complete transfer of electrons; both the hydrogen and oxygen share their electrons to form bonds.
Complete answer: Water molecules consist of hydrogen and oxygen bonded together with each other. Water exists in all the three states gaseous, liquid and solid. It is an essential component for life.
Water molecule is polar. This means that there is an unequal distribution of electron density. It has partial positive charges near the hydrogen atoms and partial negative charge near the oxygen atoms due to the unshared electron pairs. Presence of such charges on each of these atoms gives a water molecule a net dipole moment. Water molecules are difficult to separate. This is because the electrical attraction between water molecules caused by this dipole pulls the individual atoms hydrogen and oxygen close together, making it more difficult to separate them all.
The structure of molecules is very simple. Each hydrogen and oxygen molecule are bonded to each other with covalent bonds. The structure of water molecule is shown below:

Oxygen has two pair electrons which are not shared. These are lone pairs. Thus, there are$4$electrons surrounding the oxygen atom, two pairs are involved in covalent bonds with the hydrogen atom and two unshared pairs on the opposite side of the oxygen atom.
Therefore the correct option is \[A.\;\;\;\;\;4\].
Note:
Valence shell is the outermost shell of every element. They take part in bonding with the other atoms. Valence shell holds up a fixed number of electrons. The electrons in the oxygen atom which are not being used up for bonding are known as lone pairs. There is covalent bond formation in water molecules as there is not complete transfer of electrons; both the hydrogen and oxygen share their electrons to form bonds.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

How was the Civil Disobedience Movement different from class 12 social science CBSE




