
What is the electron dot structure of hydrogen chloride?
Answer
513.9k+ views
Hint: We need to know that the hydrogen chloride is also known as hydrochloric acid. It is composed of two atoms hydrogen and chlorine. The simplest covalent bond is formed between two hydrogen atoms. Each hydrogen atom has a single electron, and each needs two electrons for a full outer shell.
Complete answer:
We have to remember that the hydrogen molecule \[{H_2}\] consists of two hydrogen atoms by sharing the two valence electrons. Hydrogen can also form covalent bonds with other atoms as well. For example, hydrogen and chlorine each need one more electron to achieve a noble gas configuration. By sharing valence electrons (each atom donates one), the stable hydrogen chloride i.e. \[{\text{HCl}}\] molecule is formed.
Electron dot structure of hydrogen chloride can be represented as:
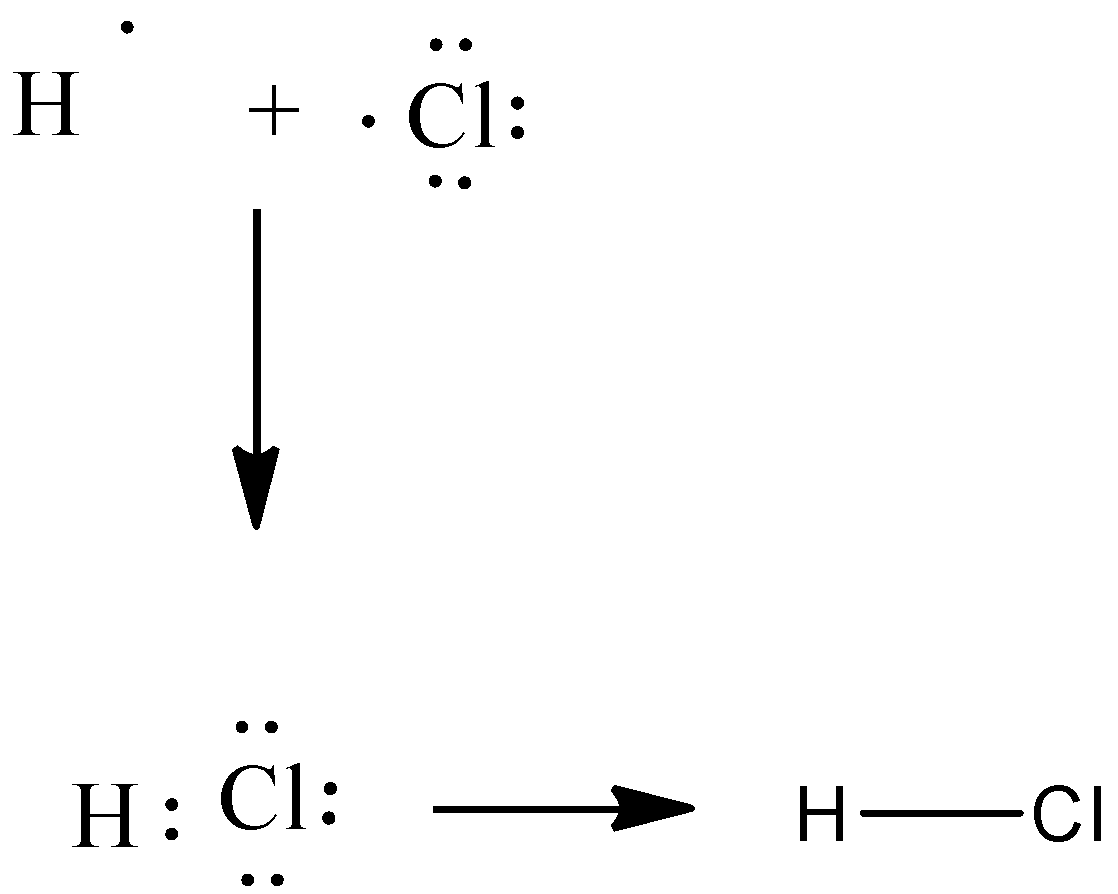
Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom \[{\text{H}}\] and a chlorine atom \[{\text{Cl}}\] connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. \[{\text{H}}\] have $2$ valence electrons as a result of sharing with \[{\text{Cl}}\] and the \[{\text{Cl}}\] has $8$ valence electrons as a result of sharing. Hydrogen chloride is an acid which is commonly called hydrochloric acid. The geometry of this molecule is linear and has $180^\circ $.
Note:
We need to remember that the chlorine atom pulls on the bonded pair of electrons harder than the hydrogen atom. This means that the electrons are not shared evenly. Some students may begin to speculate that this would cause a partial charge and that \[{\text{HCl}}\] would therefore be attracted to a charged wand.
Complete answer:
We have to remember that the hydrogen molecule \[{H_2}\] consists of two hydrogen atoms by sharing the two valence electrons. Hydrogen can also form covalent bonds with other atoms as well. For example, hydrogen and chlorine each need one more electron to achieve a noble gas configuration. By sharing valence electrons (each atom donates one), the stable hydrogen chloride i.e. \[{\text{HCl}}\] molecule is formed.
Electron dot structure of hydrogen chloride can be represented as:

Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom \[{\text{H}}\] and a chlorine atom \[{\text{Cl}}\] connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. \[{\text{H}}\] have $2$ valence electrons as a result of sharing with \[{\text{Cl}}\] and the \[{\text{Cl}}\] has $8$ valence electrons as a result of sharing. Hydrogen chloride is an acid which is commonly called hydrochloric acid. The geometry of this molecule is linear and has $180^\circ $.
Note:
We need to remember that the chlorine atom pulls on the bonded pair of electrons harder than the hydrogen atom. This means that the electrons are not shared evenly. Some students may begin to speculate that this would cause a partial charge and that \[{\text{HCl}}\] would therefore be attracted to a charged wand.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




