
What is the electron configuration for $Z{{n}^{2+}}$ ?
Answer
540.9k+ views
Hint: Zinc has an atomic number of 30. It has the atomic number of 33. Zinc is the element of the d-block. Aufbau proposed the filling of electrons. The electrons are filled in s, p, d, and f orbitals, according to the levels of energy in various shells.
Complete answer: Electronic configuration of any element tells us the total number of electrons in that atom or the atomic number of that element. Electronic configuration of any element consists of filling the orbital with electrons. The filling of electrons in various orbitals is according to a principle of Aufbau. The filling takes place in s, p, d, and f subshells. These subshells are written along with the number of shells, like 1,2, or 3.
The diagrammatic representation of the Aufbau diagram is as follows:
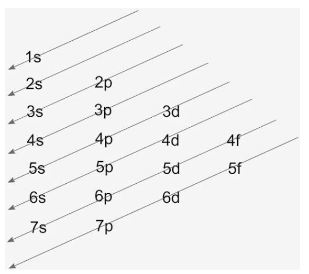
The arrows denote the order of the filling of electrons. s can accommodate 2 electrons, p can have 6, d can have 10, while f can have 14 electrons filled.
Now, given is the element $Z{{n}^{2+}}$, which is zinc with 2+ charges. The 2+ charge denotes that zinc has loosened 2 electrons from its valence shell. The atomic number of zinc is 30, so 30 electrons are present in its neutral state, so configuration in neutral state will be:
Zn =$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}$ , this is the electronic configuration of Zinc.
It can also be written by writing a noble gas (argon) and then writing the remaining electrons as,
Zn = [Ar]$3{{d}^{10}}4{{s}^{2}}$, argon with 18 electrons is added and remaining electrons are written as it is.
Now, zinc loses 2 electrons to form $Z{{n}^{2+}}$, so removing 2 electrons from the $4{{s}^{2}}$ becomes total 28 electrons, so the configuration of $Z{{n}^{2+}}$ becomes,
$Z{{n}^{2+}}$= $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}$ or [Ar]$3{{d}^{10}}$
Hence, the electronic configuration of $Z{{n}^{2+}}$ is written as $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}$ or [Ar]$3{{d}^{10}}$
Note: Another way to show the electronic configuration is writing it without sub shells (s, p, d, f). It is written showing the electrons in shells of K, L, M, N, that can have 2, 8, 18, 32 electrons respectively. So $Z{{n}^{2+}}$ will have configuration of 2, 8, 18.
Complete answer: Electronic configuration of any element tells us the total number of electrons in that atom or the atomic number of that element. Electronic configuration of any element consists of filling the orbital with electrons. The filling of electrons in various orbitals is according to a principle of Aufbau. The filling takes place in s, p, d, and f subshells. These subshells are written along with the number of shells, like 1,2, or 3.
The diagrammatic representation of the Aufbau diagram is as follows:

The arrows denote the order of the filling of electrons. s can accommodate 2 electrons, p can have 6, d can have 10, while f can have 14 electrons filled.
Now, given is the element $Z{{n}^{2+}}$, which is zinc with 2+ charges. The 2+ charge denotes that zinc has loosened 2 electrons from its valence shell. The atomic number of zinc is 30, so 30 electrons are present in its neutral state, so configuration in neutral state will be:
Zn =$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}$ , this is the electronic configuration of Zinc.
It can also be written by writing a noble gas (argon) and then writing the remaining electrons as,
Zn = [Ar]$3{{d}^{10}}4{{s}^{2}}$, argon with 18 electrons is added and remaining electrons are written as it is.
Now, zinc loses 2 electrons to form $Z{{n}^{2+}}$, so removing 2 electrons from the $4{{s}^{2}}$ becomes total 28 electrons, so the configuration of $Z{{n}^{2+}}$ becomes,
$Z{{n}^{2+}}$= $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}$ or [Ar]$3{{d}^{10}}$
Hence, the electronic configuration of $Z{{n}^{2+}}$ is written as $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}$ or [Ar]$3{{d}^{10}}$
Note: Another way to show the electronic configuration is writing it without sub shells (s, p, d, f). It is written showing the electrons in shells of K, L, M, N, that can have 2, 8, 18, 32 electrons respectively. So $Z{{n}^{2+}}$ will have configuration of 2, 8, 18.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




