
During debromination of meso-dibromobutane, the major compound formed is:
A. n-Butane
B. 1-Butene
C. cis-2-Butene
D. trans-2-Butene
Answer
567.9k+ views
Hint: We know that debromination is the reaction in which carbon bromine bonds undergo dissociation and bromine leaves the compound. Here, we have to identify the products formed in the debromination reaction.
Complete step by step answer:
First we have to draw the structure of meso-dibromobutane.
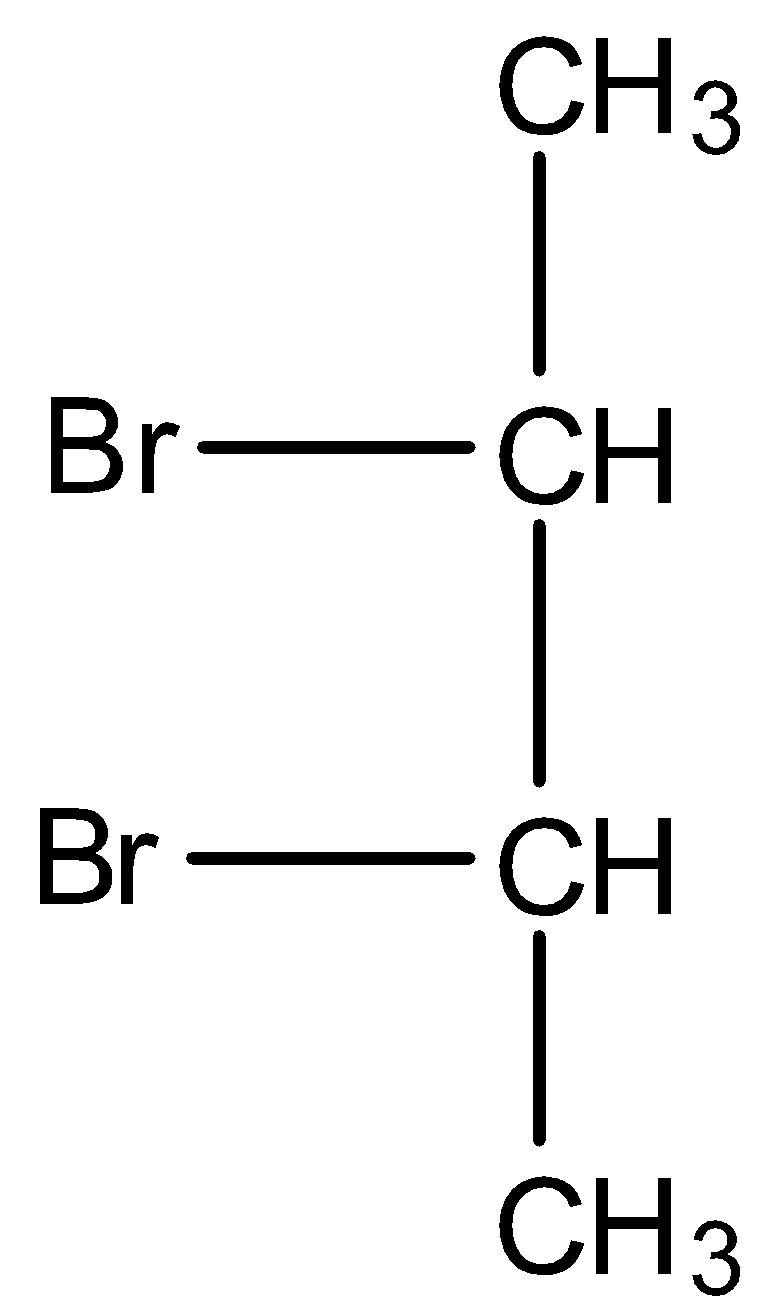
In the debromination reaction of meso-dibromobutane, the two bromine atoms leave the molecule and to stabilise the compound forms a double bond.
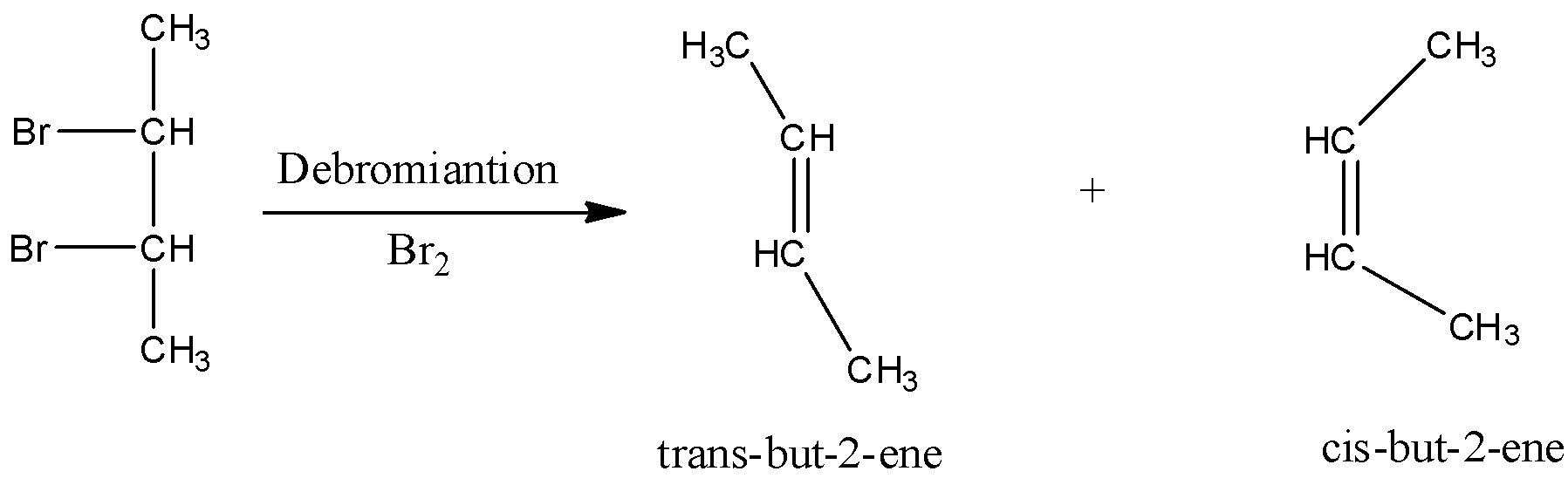
The two products namely cis-but-2-ene and trans-but-2-ene formed in the reaction. Now we have to identify the major product of the reaction. In cis-but-2-ene, the two methyl substituents are close to each other which results the steric effect. Due to this, cis-but-2-ene is unstable. In trans-but-2-ene, the two methyl substituents are not near to each other therefore steric effect is less. So, trans-2-butene is more stable. As trans-2-butene is more stable than cis-but-2-ene, it is the major product.
So, the correct answer is Option D.
Note: Let’s learn the dehalogenation reaction in detail. Debromination reaction is a type of dehalogenation reaction that involves the cleavage of C-halogen bonds to form products. There are two types of dehalogenation reaction, reductive dehalogenation and hydrohalogenation. The rate of dehalogenation reaction depends on the substrate type, oxidation state of metal and the reducing agents used during the reaction.
It is to be noted that a meso compound is a molecule possessing more than one identical stereocenter and an identical or superimposable mirror image. They are considered as achiral compounds and they contain identical mirror images.
Complete step by step answer:
First we have to draw the structure of meso-dibromobutane.

In the debromination reaction of meso-dibromobutane, the two bromine atoms leave the molecule and to stabilise the compound forms a double bond.

The two products namely cis-but-2-ene and trans-but-2-ene formed in the reaction. Now we have to identify the major product of the reaction. In cis-but-2-ene, the two methyl substituents are close to each other which results the steric effect. Due to this, cis-but-2-ene is unstable. In trans-but-2-ene, the two methyl substituents are not near to each other therefore steric effect is less. So, trans-2-butene is more stable. As trans-2-butene is more stable than cis-but-2-ene, it is the major product.
So, the correct answer is Option D.
Note: Let’s learn the dehalogenation reaction in detail. Debromination reaction is a type of dehalogenation reaction that involves the cleavage of C-halogen bonds to form products. There are two types of dehalogenation reaction, reductive dehalogenation and hydrohalogenation. The rate of dehalogenation reaction depends on the substrate type, oxidation state of metal and the reducing agents used during the reaction.
It is to be noted that a meso compound is a molecule possessing more than one identical stereocenter and an identical or superimposable mirror image. They are considered as achiral compounds and they contain identical mirror images.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




