
During change of ${{\text{O}}_{2}}$ to ${{\text{O}}_{2}}^{-}$ ion, the electron adds on which one of the following orbitals?
$\begin{align}
& \text{A}\text{. }\pi \text{ orbital} \\
& \text{B}\text{. }{{\sigma }^{*}}\text{orbital} \\
& \text{C}\text{. }\sigma \text{ orbital} \\
& \text{D}\text{. }{{\pi }^{*}}\text{ orbital} \\
\end{align}$
Answer
568.5k+ views
Hint: The question deals with diatomic molecule which are ${{\text{O}}_{2}}$ and ${{\text{O}}_{2}}^{-}$. Diatomic molecules and their configuration comes under Molecular orbital theory. It is a method that is used to describe the electronic structure of diatomic molecules using quantum mechanics.
Complete Solution:
The addition of electrons in which an orbital cannot be explained without knowing what is molecular orbital theory and its general configuration. Let us first see the definition; This theory uses a linear combination of atomic orbitals which represents molecular orbitals that result from bonds between atomic orbitals. It is categorized into three types: bonding, antibonding and non-bonding.
Oxygen molecule or ${{\text{O}}_{2}}$- The electronic configuration of oxygen (Z = 8) in its ground state is $1{{\text{s}}^{2}}2{{\text{s}}^{2}}2{{\text{p}}^{4}}$. Oxygen atoms have 8 electrons thus; there are 16 electrons in ${{\text{O}}_{2}}$ molecule. The electronic configuration of ${{\text{O}}_{2}}$:
${{\text{O}}_{2}}$:${{\sigma }_{\text{1s}}}^{2}{{\sigma }^{*}}{{_{1\text{s}}}^{2}}{{\sigma }_{2\text{s}}}^{2}{{\sigma }^{*}}{{_{2\text{s}}}^{2}}{{\sigma }_{2\text{pz}}}^{2}{{\pi }_{2\text{px}}}^{2}{{\pi }_{2\text{py}}}^{2}{{\pi }^{*}}{{_{2\text{px}}}^{1}}{{\pi }^{*}}{{_{2\text{py}}}^{1}}$; the electrons in molecular orbitals are filled according to Hund’s Rule and Aufbau rule.
- Hund’s Rule: Each orbital in a subshell is filled with one electron before its following orbital is filled again and all electrons which are singly filled in the orbitals have the same spin. See the difference between the configurations,
$\text{CORRECT}-\text{ }\begin{matrix}
\uparrow & \uparrow & \uparrow & {} & {} \\
\end{matrix}
\\
\text{INCORRECT}-\text{ }\begin{matrix}
\uparrow \downarrow & \uparrow & {} & {} & {} \\
\end{matrix} $
- Aufbau Rule- It states that in the ground state of an atom; electrons fill orbitals of the lowest energy levels before occupying higher levels. Like 3d is filled before 4s. Its molecular orbital diagram is (p-orbital):
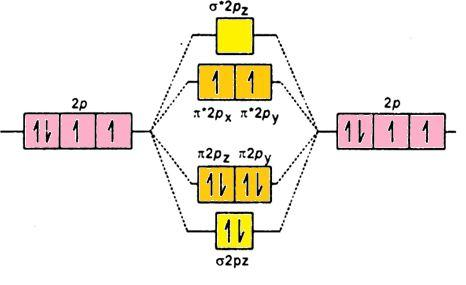
Let us see the electronic configuration of${{\text{O}}_{2}}^{-}$ it has total ( 8 + 8 + 1) electrons in it that is total 17 electrons:
${{\text{O}}_{2}}^{-}$: ${{\sigma }_{\text{1s}}}^{2}{{\sigma }^{*}}{{_{1\text{s}}}^{2}}{{\sigma }_{2\text{s}}}^{2}{{\sigma }^{*}}{{_{2\text{s}}}^{2}}{{\sigma }_{2\text{pz}}}^{2}{{\pi }_{2\text{px}}}^{2}{{\pi }_{2\text{py}}}^{2}{{\pi }^{*}}{{_{2\text{px}}}^{2}}{{\pi }^{*}}{{_{2\text{py}}}^{1}}$: addition of electron took place in the antibonding orbital which is $\pi _{2\text{px}}^{*}$, the number of electrons has increased from 1 to 2 in that orbital. Its molecular orbital diagram is (p-orbital):
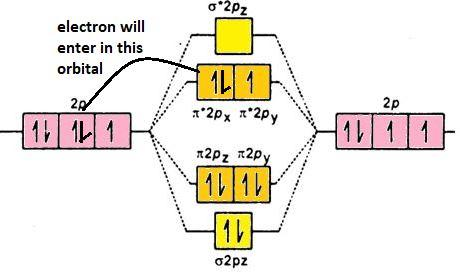
Thus, the correct answer to this question is the incoming electron added to ${{\pi }^{*}}$
So, the correct answer is “Option D”.
Note: The electronic configuration for elements having electrons greater than 14 electrons is different with the configuration of elements having electrons less than equal to 14. Let us see the electronic configuration:
(1) Greater than 14 electrons: ${{\sigma }_{\text{1s}}}{{\sigma }^{*}}_{1\text{s}}{{\sigma }_{2\text{s}}}{{\sigma }^{*}}_{2\text{s}}{{\sigma }_{2\text{pz}}}{{\pi }_{2\text{px}}}{{\pi }_{2\text{py}}}{{\pi }^{*}}_{2\text{px}}{{\pi }^{*}}_{2\text{py}}$
(2) Electrons less than equal to 14 electrons: ${{\sigma }_{\text{1s}}}{{\sigma }^{*}}_{1\text{s}}{{\sigma }_{2\text{s}}}{{\sigma }^{*}}_{2\text{s}}{{\pi }_{2\text{px}}}{{\pi }_{2\text{py}}}{{\pi }^{*}}_{2\text{px}}{{\sigma }_{2\text{pz}}}{{\pi }^{*}}_{2\text{py}}\sigma _{2\text{pz}}^{*}$
Complete Solution:
The addition of electrons in which an orbital cannot be explained without knowing what is molecular orbital theory and its general configuration. Let us first see the definition; This theory uses a linear combination of atomic orbitals which represents molecular orbitals that result from bonds between atomic orbitals. It is categorized into three types: bonding, antibonding and non-bonding.
Oxygen molecule or ${{\text{O}}_{2}}$- The electronic configuration of oxygen (Z = 8) in its ground state is $1{{\text{s}}^{2}}2{{\text{s}}^{2}}2{{\text{p}}^{4}}$. Oxygen atoms have 8 electrons thus; there are 16 electrons in ${{\text{O}}_{2}}$ molecule. The electronic configuration of ${{\text{O}}_{2}}$:
${{\text{O}}_{2}}$:${{\sigma }_{\text{1s}}}^{2}{{\sigma }^{*}}{{_{1\text{s}}}^{2}}{{\sigma }_{2\text{s}}}^{2}{{\sigma }^{*}}{{_{2\text{s}}}^{2}}{{\sigma }_{2\text{pz}}}^{2}{{\pi }_{2\text{px}}}^{2}{{\pi }_{2\text{py}}}^{2}{{\pi }^{*}}{{_{2\text{px}}}^{1}}{{\pi }^{*}}{{_{2\text{py}}}^{1}}$; the electrons in molecular orbitals are filled according to Hund’s Rule and Aufbau rule.
- Hund’s Rule: Each orbital in a subshell is filled with one electron before its following orbital is filled again and all electrons which are singly filled in the orbitals have the same spin. See the difference between the configurations,
$\text{CORRECT}-\text{ }\begin{matrix}
\uparrow & \uparrow & \uparrow & {} & {} \\
\end{matrix}
\\
\text{INCORRECT}-\text{ }\begin{matrix}
\uparrow \downarrow & \uparrow & {} & {} & {} \\
\end{matrix} $
- Aufbau Rule- It states that in the ground state of an atom; electrons fill orbitals of the lowest energy levels before occupying higher levels. Like 3d is filled before 4s. Its molecular orbital diagram is (p-orbital):

Let us see the electronic configuration of${{\text{O}}_{2}}^{-}$ it has total ( 8 + 8 + 1) electrons in it that is total 17 electrons:
${{\text{O}}_{2}}^{-}$: ${{\sigma }_{\text{1s}}}^{2}{{\sigma }^{*}}{{_{1\text{s}}}^{2}}{{\sigma }_{2\text{s}}}^{2}{{\sigma }^{*}}{{_{2\text{s}}}^{2}}{{\sigma }_{2\text{pz}}}^{2}{{\pi }_{2\text{px}}}^{2}{{\pi }_{2\text{py}}}^{2}{{\pi }^{*}}{{_{2\text{px}}}^{2}}{{\pi }^{*}}{{_{2\text{py}}}^{1}}$: addition of electron took place in the antibonding orbital which is $\pi _{2\text{px}}^{*}$, the number of electrons has increased from 1 to 2 in that orbital. Its molecular orbital diagram is (p-orbital):

Thus, the correct answer to this question is the incoming electron added to ${{\pi }^{*}}$
So, the correct answer is “Option D”.
Note: The electronic configuration for elements having electrons greater than 14 electrons is different with the configuration of elements having electrons less than equal to 14. Let us see the electronic configuration:
(1) Greater than 14 electrons: ${{\sigma }_{\text{1s}}}{{\sigma }^{*}}_{1\text{s}}{{\sigma }_{2\text{s}}}{{\sigma }^{*}}_{2\text{s}}{{\sigma }_{2\text{pz}}}{{\pi }_{2\text{px}}}{{\pi }_{2\text{py}}}{{\pi }^{*}}_{2\text{px}}{{\pi }^{*}}_{2\text{py}}$
(2) Electrons less than equal to 14 electrons: ${{\sigma }_{\text{1s}}}{{\sigma }^{*}}_{1\text{s}}{{\sigma }_{2\text{s}}}{{\sigma }^{*}}_{2\text{s}}{{\pi }_{2\text{px}}}{{\pi }_{2\text{py}}}{{\pi }^{*}}_{2\text{px}}{{\sigma }_{2\text{pz}}}{{\pi }^{*}}_{2\text{py}}\sigma _{2\text{pz}}^{*}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




