
During acetylation of glucose it needs moles of acetic anhydride. The value of would be:
A. 3
B. 5
C. 4
D. 1
Answer
587.4k+ views
Hint: Acetylation is a type of reaction in which a hydrogen atom is substituted by an acetyl ($C{H_3}C = O$) group in a compound. When the hydrogen of hydroxide group is replaced by acetyl group then ester will be formed as a product.
Complete solution:
First let us understand about glucose and its properties.
Glucose is the most common monosaccharide .It is known as Dextrose because it occurs in nature principally as the optically active dextrorotatory isomers.
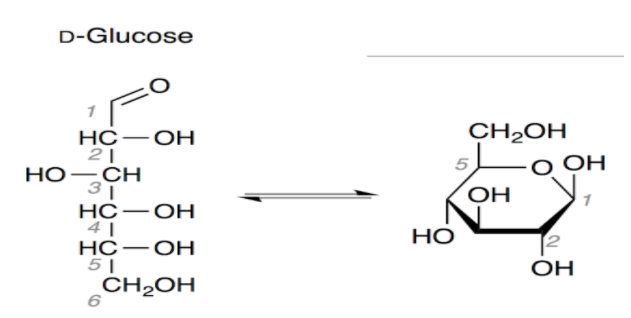
The six membered structure of glucose is called pyranose structure. When glucose is acylated with acid halide or acetic anhydride gives pentaacetate which confirms the presence of five –OH groups.
You have seen above in the structure of glucose that there are five hydrogens which are attached to oxygen and they can be replaced by an acetyl group.
So there is a need of 5 moles of acetic anhydride needed in acetylation of glucose.
Therefore the correct option: B. 5
Additional Information:
Glucose is white crystalline solid having a melting point of ${146^0}$C. When the glucose molecule is crystallized with cold water, it forms glucose monohydrate of melting point ${86^0}$C. It is extremely soluble in water, only sparingly soluble in ethanol and insoluble in ether. It is an optically active compound.
Note:
Remember acetylation takes place only on the hydrogen which is attached to oxygen. Rate of reaction can vary on different hydroxyl groups. Also remember the acylation and acetylation are two different types of reactions.
Complete solution:
First let us understand about glucose and its properties.
Glucose is the most common monosaccharide .It is known as Dextrose because it occurs in nature principally as the optically active dextrorotatory isomers.

The six membered structure of glucose is called pyranose structure. When glucose is acylated with acid halide or acetic anhydride gives pentaacetate which confirms the presence of five –OH groups.
You have seen above in the structure of glucose that there are five hydrogens which are attached to oxygen and they can be replaced by an acetyl group.
So there is a need of 5 moles of acetic anhydride needed in acetylation of glucose.
Therefore the correct option: B. 5
Additional Information:
Glucose is white crystalline solid having a melting point of ${146^0}$C. When the glucose molecule is crystallized with cold water, it forms glucose monohydrate of melting point ${86^0}$C. It is extremely soluble in water, only sparingly soluble in ethanol and insoluble in ether. It is an optically active compound.
Note:
Remember acetylation takes place only on the hydrogen which is attached to oxygen. Rate of reaction can vary on different hydroxyl groups. Also remember the acylation and acetylation are two different types of reactions.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




