
How do you draw this cyclic-pentane? \[4 - ethyl - 2 - isopropyl - 1 - methylcyclopentane\]
Answer
499.2k+ views
Hint: To solve such a question we first break down the given compound in parts then we will try to find the different functional group attached to it. When we start drawing the structure then we first find the longest carbon chain and attach other functions to this chain.
We will do the same in this question as it is cyclic pentane so it will be our longest carbon chain and we will go further.
Complete answer:
Let's have a look at this in more detail.
Your first task is to locate the parent chain, which is the carbon chain with the longest length.
That's simple because the structure's name includes the word cyclopentane in the last portion of the name. The prefix cyclo- indicates that the parent chain is cyclic. Pentane is a 5 carbon alkane, and the prefix cyclo- indicates that the parent chain is cyclic. It appears like this when there aren't any alkyl chains linked.
After that, you must determine the substituents. Knowing that cyclopentane is the parent chain, we can regard the rest of the molecules as substituents. Let's put them down on paper.
4-ethyl
2-isopropyl
1-methyl
If we take our cyclopentane and count all of the carbons, we get
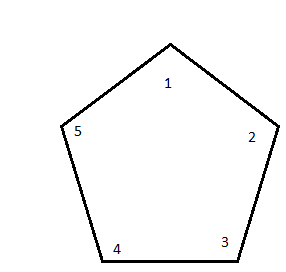
The substituents can then be attached one by one, starting in numerical order.

After that, we'll look into 2-isopropyl. At carbon number 2, we have an isopropyl group connected. Putting this alkyl group in its proper place gives us the following structure so far:
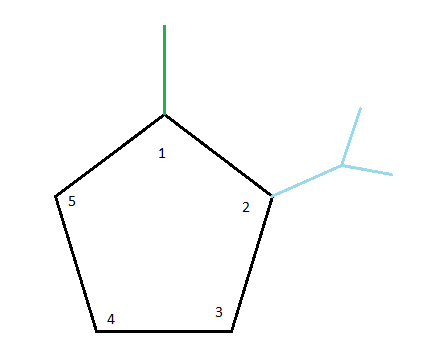
Finally, while considering 4-ethyl, we place an ethyl group at carbon number 4. As a result, our final structure will look like this:
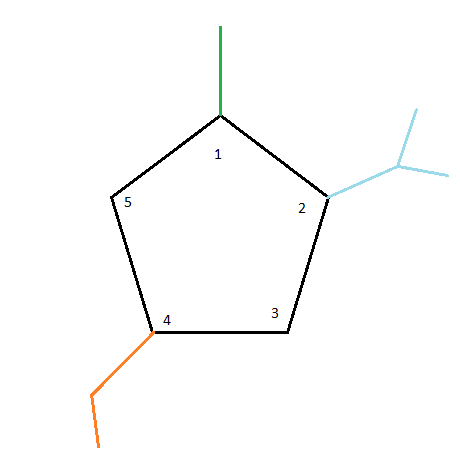
\[4 - ethyl - 2 - isopropyl - 1 - methylcyclopentane\]
Note:
Carbonyls ( $C = O$ ), alcohols ( \[ - OH\] ), carboxylic acids ( \[C{O_2}H\] ), ester (\[C{O_2}R\] ), and amines are the most frequent functional groups in organic chemistry ( $N{H_2}$ ). It's crucial to understand functional groups and the physical and chemical qualities they provide.
We will do the same in this question as it is cyclic pentane so it will be our longest carbon chain and we will go further.
Complete answer:
Let's have a look at this in more detail.
Your first task is to locate the parent chain, which is the carbon chain with the longest length.
That's simple because the structure's name includes the word cyclopentane in the last portion of the name. The prefix cyclo- indicates that the parent chain is cyclic. Pentane is a 5 carbon alkane, and the prefix cyclo- indicates that the parent chain is cyclic. It appears like this when there aren't any alkyl chains linked.

After that, you must determine the substituents. Knowing that cyclopentane is the parent chain, we can regard the rest of the molecules as substituents. Let's put them down on paper.
4-ethyl
2-isopropyl
1-methyl
If we take our cyclopentane and count all of the carbons, we get

The substituents can then be attached one by one, starting in numerical order.

After that, we'll look into 2-isopropyl. At carbon number 2, we have an isopropyl group connected. Putting this alkyl group in its proper place gives us the following structure so far:

Finally, while considering 4-ethyl, we place an ethyl group at carbon number 4. As a result, our final structure will look like this:

\[4 - ethyl - 2 - isopropyl - 1 - methylcyclopentane\]
Note:
Carbonyls ( $C = O$ ), alcohols ( \[ - OH\] ), carboxylic acids ( \[C{O_2}H\] ), ester (\[C{O_2}R\] ), and amines are the most frequent functional groups in organic chemistry ( $N{H_2}$ ). It's crucial to understand functional groups and the physical and chemical qualities they provide.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




