
Draw the valence shell molecular orbital diagram of the oxygen molecule and predict its magnetic nature.
Answer
573.6k+ views
Hint: The magnetic property of a molecule can be explained based on the molecular orbital theory. The molecule which does not contain the unpaired electron is known as the paramagnetic. The molecule which has all-electron paid-up does not contribute towards the magnetic property. It is diamagnetic in nature. To solve such a problem write down the MOT diagram of molecules.
Complete step by step solution:
Let’s first draw the MOT of the oxygen molecule. The electronic configuration of oxygen atom is as shown below,
$\text{ O = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}_{\text{x}}}^{\text{2}}\text{=2}{{\text{p}}_{\text{y}}}^{\text{1}}\text{=2}{{\text{p}}_{\text{z}}}^{\text{1}}\text{ }$
Thus the oxygen molecule ${{\text{O}}_{\text{2}}}$ contains 16 electrons. The MOT is as shown below,
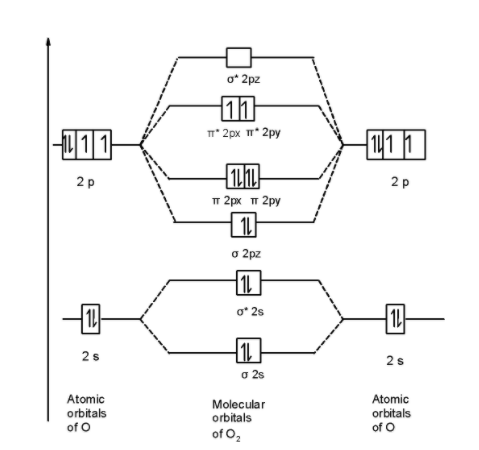
First of all, we can write the molecular orbital configuration of ${{\text{O}}_{\text{2}}}$ the molecule. In a ${{\text{O}}_{\text{2}}}$ molecule, there are a total of 16 electrons. The molecular orbital configuration of ${{\text{O}}_{\text{2}}}$ the molecule is as follows:
\[\]\[\text{ }\!\!\sigma\!\!\text{ 1}{{\text{s}}^{\text{2}}}\text{,}{{\text{ }\!\!\sigma\!\!\text{ }}^{\text{*}}}\text{1}{{\text{s}}^{\text{2}}}\text{, }\!\!\sigma\!\!\text{ 2}{{\text{s}}^{\text{2}}}\text{, }{{\text{ }\!\!\sigma\!\!\text{ }}^{\text{*}}}\text{2}{{\text{s}}^{\text{2}}}\text{, }\!\!\sigma\!\!\text{ 2}{{\text{p}}^{\text{2}}}_{z}\text{, 2p}_{\text{x}}^{\text{2}}\text{ }\!\!\pi\!\!\text{ = 2p}_{\text{y}}^{\text{2}}\text{ }\!\!\pi\!\!\text{ , 2p}_{\text{x}}^{1}{{\text{ }\!\!\pi\!\!\text{ }}^{\text{*}}}\text{=2p}_{\text{y}}^{1}{{\text{ }\!\!\pi\!\!\text{ }}^{\text{*}}}\]
There are 10 bonding and 6 nonbonding electrons in the orbitals according to the molecular orbital configuration.
Therefore, $\text{Bond order =}\dfrac{\text{1}}{\text{2}}\left[ \text{Bonding-antibonding} \right]$
= $\dfrac{1}{2}\left[ 10-6 \right]=\dfrac{1}{2}\left( 4 \right)=2$
Thus, the bond order ${{\text{O}}_{\text{2}}}$ is 2.
We know that if a molecule has paired electrons in molecular orbits then it shows diamagnetic properties however if a molecule has unpaired electrons in its MO diagram then it exhibits paramagnetic properties.
Here, from the above MO diagram we observe two unpaired electrons in the $\text{ }\!\!\pi\!\!\text{ *2py }$ and $\text{ }\!\!\pi\!\!\text{ *2px }$molecular orbitals. Thus due to two unpaired electrons in the MO diagram oxygen molecule is paramagnetic in nature.
Note: Note that if we look at the Lewis dot structure we see that electrons in two oxygens are paired thus oxygen molecules should be diamagnetic.

But remember that the Lewis dot structure of the oxygen molecule is misleading. Magnetic properties are well studied by the MOT diagram.
Complete step by step solution:
Let’s first draw the MOT of the oxygen molecule. The electronic configuration of oxygen atom is as shown below,
$\text{ O = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}_{\text{x}}}^{\text{2}}\text{=2}{{\text{p}}_{\text{y}}}^{\text{1}}\text{=2}{{\text{p}}_{\text{z}}}^{\text{1}}\text{ }$
Thus the oxygen molecule ${{\text{O}}_{\text{2}}}$ contains 16 electrons. The MOT is as shown below,

First of all, we can write the molecular orbital configuration of ${{\text{O}}_{\text{2}}}$ the molecule. In a ${{\text{O}}_{\text{2}}}$ molecule, there are a total of 16 electrons. The molecular orbital configuration of ${{\text{O}}_{\text{2}}}$ the molecule is as follows:
\[\]\[\text{ }\!\!\sigma\!\!\text{ 1}{{\text{s}}^{\text{2}}}\text{,}{{\text{ }\!\!\sigma\!\!\text{ }}^{\text{*}}}\text{1}{{\text{s}}^{\text{2}}}\text{, }\!\!\sigma\!\!\text{ 2}{{\text{s}}^{\text{2}}}\text{, }{{\text{ }\!\!\sigma\!\!\text{ }}^{\text{*}}}\text{2}{{\text{s}}^{\text{2}}}\text{, }\!\!\sigma\!\!\text{ 2}{{\text{p}}^{\text{2}}}_{z}\text{, 2p}_{\text{x}}^{\text{2}}\text{ }\!\!\pi\!\!\text{ = 2p}_{\text{y}}^{\text{2}}\text{ }\!\!\pi\!\!\text{ , 2p}_{\text{x}}^{1}{{\text{ }\!\!\pi\!\!\text{ }}^{\text{*}}}\text{=2p}_{\text{y}}^{1}{{\text{ }\!\!\pi\!\!\text{ }}^{\text{*}}}\]
There are 10 bonding and 6 nonbonding electrons in the orbitals according to the molecular orbital configuration.
Therefore, $\text{Bond order =}\dfrac{\text{1}}{\text{2}}\left[ \text{Bonding-antibonding} \right]$
= $\dfrac{1}{2}\left[ 10-6 \right]=\dfrac{1}{2}\left( 4 \right)=2$
Thus, the bond order ${{\text{O}}_{\text{2}}}$ is 2.
We know that if a molecule has paired electrons in molecular orbits then it shows diamagnetic properties however if a molecule has unpaired electrons in its MO diagram then it exhibits paramagnetic properties.
Here, from the above MO diagram we observe two unpaired electrons in the $\text{ }\!\!\pi\!\!\text{ *2py }$ and $\text{ }\!\!\pi\!\!\text{ *2px }$molecular orbitals. Thus due to two unpaired electrons in the MO diagram oxygen molecule is paramagnetic in nature.
Note: Note that if we look at the Lewis dot structure we see that electrons in two oxygens are paired thus oxygen molecules should be diamagnetic.

But remember that the Lewis dot structure of the oxygen molecule is misleading. Magnetic properties are well studied by the MOT diagram.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




