
Draw the structure of TNT, an explosive.
Answer
573.6k+ views
Hint: TNT is explosive as contains the elements oxygen nitrogen, and carbon, which tells us that when the material burns it could produce highly stable substances (\[CO, C{{O}_{2}}, {{N}_{2}}\]) which has strong bonds, which in turn releases a huge amount of energy.
Another reason TNT is explosive, is that it is chemically very unstable. The alternate nitro groups are so closely packed that they experience a huge amount of strain and steric hindrance.
Complete step by step answer:
TNT or \[2,4,6-\text{trinitrotoluene}\], is a type of chemical compound which can be represented by the formula \[{{C}_{6}}{{H}_{2}}{{(N{{O}_{2}})}_{3}}C{{H}_{3}}\]. It is a yellow solid which is is occasionally used as a reagent in synthesis of chemical compounds, but it is best known for explosive nature of the material with appropriate handling properties
The structure of TNT, or trinitrotoluene consists of three
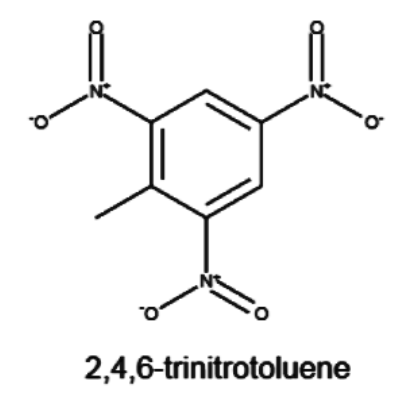
We will do a thorough step by step analysis of the name, and hence justify the structure which is given above.
As we can tell, from the name of the compound tri- means three and nitro means nitro group, which contains a nitrogen in which two oxygen groups are attached. The three nitro groups are present in alternate carbons of the benzene ring. However, the numbering of TNT is given from the perspective of the alkyl group, which makes the root name of the benzene ring, toluene. The presence of three nitro groups makes it very reactive hence the explosive nature.
When the TNT undergoes detonation, experiences decomposition equivalent to the reaction
\[2{ }{{C}_{7}}{{H}_{5}}{{N}_{3}}{{O}_{6}}~\to { }3{ }{{N}_{2}}~+{ }5{ }{{H}_{2}}~+{ }12{ }CO{ }+{ }2{ }C\]
Detonation is a type of reaction of combustion which involves a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it.
TNT is one of the most frequently used explosives for mining, military, and industrial applications. TNT could be used in combination with hydraulic fracturing, a process which is used to recover oil and gas from formation of shale. This technique usually involves displacing and detonating nitro-glycerine in hydraulically induced breaks followed by wellbore shots using pelletized TNT.
Note: TNT is also poisonous in nature, and contact with skin can cause irritation, as it causes the skin to turn into bright yellow-orange colour.
TNT does not explode spontaneously, and in fact it can be treated quite roughly. In order to initiate the explosion, first TNT must be detonated using a pressure wave from another explosion which is more easily induced from another explosive called a detonator.
Another reason TNT is explosive, is that it is chemically very unstable. The alternate nitro groups are so closely packed that they experience a huge amount of strain and steric hindrance.
Complete step by step answer:
TNT or \[2,4,6-\text{trinitrotoluene}\], is a type of chemical compound which can be represented by the formula \[{{C}_{6}}{{H}_{2}}{{(N{{O}_{2}})}_{3}}C{{H}_{3}}\]. It is a yellow solid which is is occasionally used as a reagent in synthesis of chemical compounds, but it is best known for explosive nature of the material with appropriate handling properties
The structure of TNT, or trinitrotoluene consists of three

We will do a thorough step by step analysis of the name, and hence justify the structure which is given above.
As we can tell, from the name of the compound tri- means three and nitro means nitro group, which contains a nitrogen in which two oxygen groups are attached. The three nitro groups are present in alternate carbons of the benzene ring. However, the numbering of TNT is given from the perspective of the alkyl group, which makes the root name of the benzene ring, toluene. The presence of three nitro groups makes it very reactive hence the explosive nature.
When the TNT undergoes detonation, experiences decomposition equivalent to the reaction
\[2{ }{{C}_{7}}{{H}_{5}}{{N}_{3}}{{O}_{6}}~\to { }3{ }{{N}_{2}}~+{ }5{ }{{H}_{2}}~+{ }12{ }CO{ }+{ }2{ }C\]
Detonation is a type of reaction of combustion which involves a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it.
TNT is one of the most frequently used explosives for mining, military, and industrial applications. TNT could be used in combination with hydraulic fracturing, a process which is used to recover oil and gas from formation of shale. This technique usually involves displacing and detonating nitro-glycerine in hydraulically induced breaks followed by wellbore shots using pelletized TNT.
Note: TNT is also poisonous in nature, and contact with skin can cause irritation, as it causes the skin to turn into bright yellow-orange colour.
TNT does not explode spontaneously, and in fact it can be treated quite roughly. In order to initiate the explosion, first TNT must be detonated using a pressure wave from another explosion which is more easily induced from another explosive called a detonator.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




