
Draw the structure of the Hexanal molecule.
Answer
589.5k+ views
Hint: It has an aldehyde functional group present in the molecule. The word ‘hex’ suggests the presence of six carbon atoms in the longest possible chain in the organic compound.
Complete step by step answer:
Let’s start with identifying the functional group present in the compound in order to find its structure.
- We can see that there is –al suffix in the name Hexanal. That suggests the presence of an aldehyde functional group.
- The word ‘hex’ in the Hexanal shows the presence of six carbon atoms in the structure.
- Now, we will need to find out at which place this aldehyde group will attach. Here, we are not given a specific position by showing the number of the aldehyde carbon. So, in this case, we will take this as 1-Hexanal.
- That means that the aldehyde group is attached at the first carbon of the alkyl chain. In addition, there are no substituent groups attached to the carbon chain.
- As a total of six carbons are present in the compound and no substituent groups are present in the group, we can say that there is a six carbon chain present in the molecule in which aldehyde carbon is the first carbon of the chain.
So, we can give the following structure to the Hexanal.
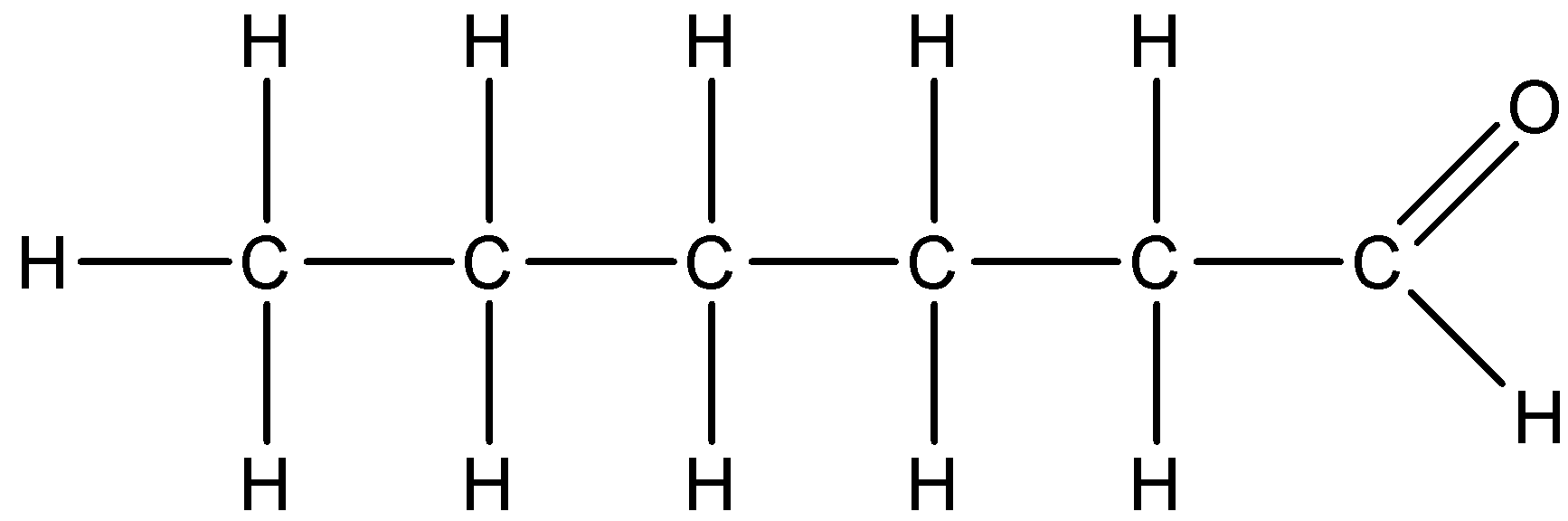
Note: Here, we are not given the position of the aldehyde functional group but we should be able to get that this is the only possible structure to a compound which has aldehyde functional group with total six carbon atoms and it does not have any substituent groups attached to the parent carbon chain.
Complete step by step answer:
Let’s start with identifying the functional group present in the compound in order to find its structure.
- We can see that there is –al suffix in the name Hexanal. That suggests the presence of an aldehyde functional group.
- The word ‘hex’ in the Hexanal shows the presence of six carbon atoms in the structure.
- Now, we will need to find out at which place this aldehyde group will attach. Here, we are not given a specific position by showing the number of the aldehyde carbon. So, in this case, we will take this as 1-Hexanal.
- That means that the aldehyde group is attached at the first carbon of the alkyl chain. In addition, there are no substituent groups attached to the carbon chain.
- As a total of six carbons are present in the compound and no substituent groups are present in the group, we can say that there is a six carbon chain present in the molecule in which aldehyde carbon is the first carbon of the chain.
So, we can give the following structure to the Hexanal.

Note: Here, we are not given the position of the aldehyde functional group but we should be able to get that this is the only possible structure to a compound which has aldehyde functional group with total six carbon atoms and it does not have any substituent groups attached to the parent carbon chain.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




