
Draw the structure of the following compound: 2, 2 – Dimethylpentane
Answer
533.4k+ views
Hint: The given compound is one of the isomers of heptane which is known as neo-heptane. It contains one quaternary carbon group at one end of the carbon chain. The molecular formula is ${{\text{C}}_{7}}{{\text{H}}_{16}}$.
Complete step by step answer: 2, 2 – Dimethylpentane is an unsaturated hydrocarbon that is made up of a long-chain of 7 carbon atoms. But the parent carbon chain or the longest carbon chain consists of only 5 carbons. The other 2 carbons are present as the methyl groups.
According to the nomenclature of the compound, the parent hydrocarbon chain is pentane and the two methyl groups are attached to the second carbon of the parent hydrocarbon chain.
To draw the structure of the given compound, start by numbering the parent carbon chain.
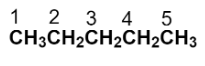
Now, we have to replace the two hydrogens with two methyl groups at the carbon numbered as 2.
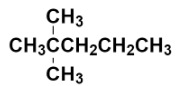
This is the structure of 2, 2 – Dimethylpentane. It is also known as neo-heptane because it is an isomer of heptane containing neo $\left( {{\left( \text{C}{{\text{H}}_{3}} \right)}_{3}}\text{C}- \right)$ group.
Hence, the structure of 2, 2 – Dimethylpentane is:
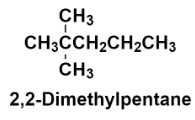
Additional information:
2, 2 – Dimethylpentane is an odorless and colorless liquid and found in a very low amount of about $0.01%$ in some crude oils
Note: 2, 2 – Dimethylpentane does not contain any tertiary carbon that is attached to three carbon atoms and one hydrogen and that is one of the reasons why it does not react with nitric acid.
Complete step by step answer: 2, 2 – Dimethylpentane is an unsaturated hydrocarbon that is made up of a long-chain of 7 carbon atoms. But the parent carbon chain or the longest carbon chain consists of only 5 carbons. The other 2 carbons are present as the methyl groups.
According to the nomenclature of the compound, the parent hydrocarbon chain is pentane and the two methyl groups are attached to the second carbon of the parent hydrocarbon chain.
To draw the structure of the given compound, start by numbering the parent carbon chain.
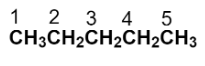
Now, we have to replace the two hydrogens with two methyl groups at the carbon numbered as 2.

This is the structure of 2, 2 – Dimethylpentane. It is also known as neo-heptane because it is an isomer of heptane containing neo $\left( {{\left( \text{C}{{\text{H}}_{3}} \right)}_{3}}\text{C}- \right)$ group.
Hence, the structure of 2, 2 – Dimethylpentane is:

Additional information:
2, 2 – Dimethylpentane is an odorless and colorless liquid and found in a very low amount of about $0.01%$ in some crude oils
Note: 2, 2 – Dimethylpentane does not contain any tertiary carbon that is attached to three carbon atoms and one hydrogen and that is one of the reasons why it does not react with nitric acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




