
Draw the structure of ethanoic acid molecule
Answer
592.8k+ views
Hint: Think about what the prefix ‘ethan-’ and the suffix ‘-oic acid’ may indicate. Think about the rules that are related to the nomenclature of those particular functional groups.
Complete answer:
The steps to draw the ethanoic acid molecule can be carried out in steps. They are as follows:
- From the name we can understand that there will be two carbon atoms. This can be figured out from the fact that ‘ethan’ means two carbon atoms that are joined by a single bond.
- The parent functional group is COOH. This can be predicted from the name of the compound itself. The compound says, ‘oic acid’. This signifies the compound should be having a carboxyl group.
- We should have an idea about the general formula of the carboxyl group, that is $R-COOH$. $R-$ refers to the alkyl group. The alkyl group will have 1 carbon and the carboxyl group will have 1 carbon which will make up the 2 carbons that are present in the molecule.
So, keeping the above points in mind, we can say that the structure of ethanoic acid is:
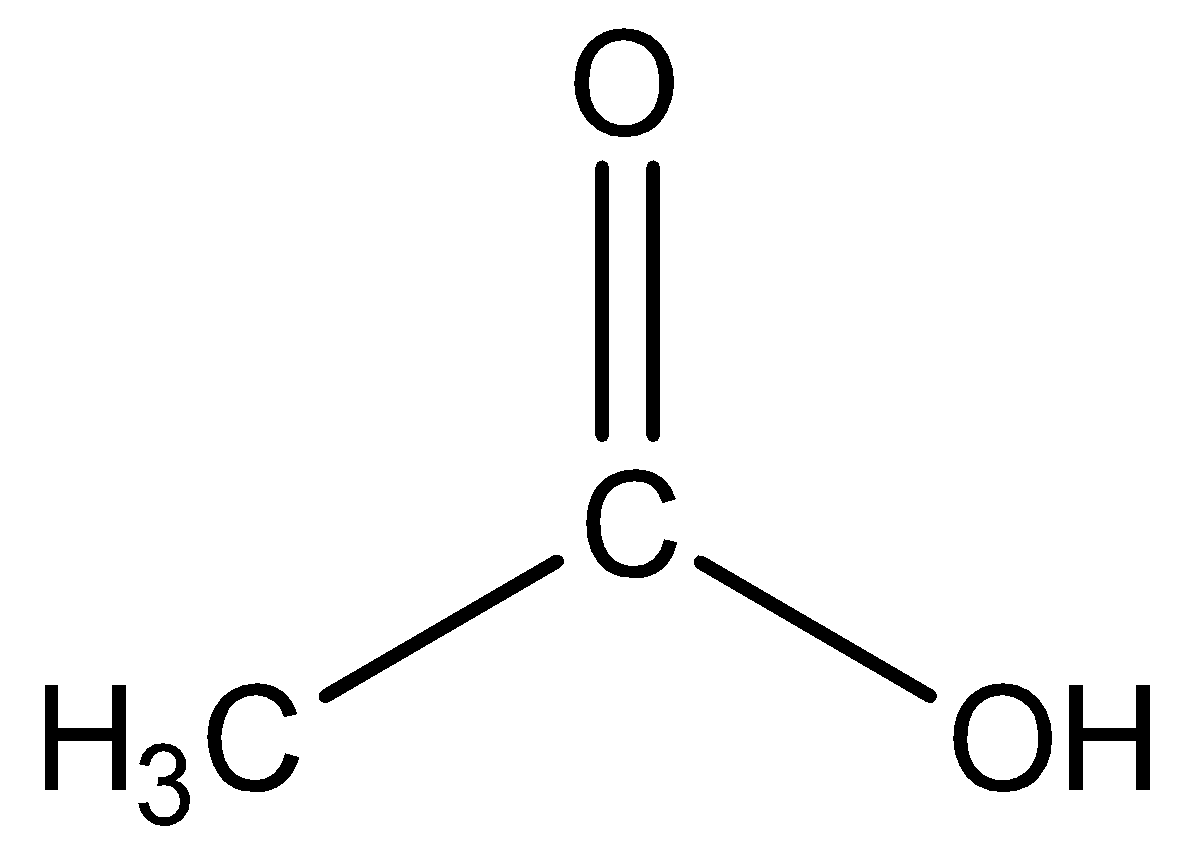
Additional Information: A basic idea about the uses of ethanoic acid or acetic acid is for the dyes, pigments, paints, etc. It is also used as a cleaning and degreasing solvent.
Ethanoic acid is a weak acid, which means it does not fully dissociate into ions in water. It is found in vinegar. Only 4 out of 100 ethanoic acid molecules dissociate in water.
Note: The commercial name of ethanoic acid is acetic acid that is found in vinegar. Do not get confused if acetic acid is mentioned instead of ethanoic acid. Remember that it is not the alkyl group attached to the carboxylic acid group that has 2 carbons but the whole molecule that has 2 carbons.
Complete answer:
The steps to draw the ethanoic acid molecule can be carried out in steps. They are as follows:
- From the name we can understand that there will be two carbon atoms. This can be figured out from the fact that ‘ethan’ means two carbon atoms that are joined by a single bond.
- The parent functional group is COOH. This can be predicted from the name of the compound itself. The compound says, ‘oic acid’. This signifies the compound should be having a carboxyl group.
- We should have an idea about the general formula of the carboxyl group, that is $R-COOH$. $R-$ refers to the alkyl group. The alkyl group will have 1 carbon and the carboxyl group will have 1 carbon which will make up the 2 carbons that are present in the molecule.
So, keeping the above points in mind, we can say that the structure of ethanoic acid is:

Additional Information: A basic idea about the uses of ethanoic acid or acetic acid is for the dyes, pigments, paints, etc. It is also used as a cleaning and degreasing solvent.
Ethanoic acid is a weak acid, which means it does not fully dissociate into ions in water. It is found in vinegar. Only 4 out of 100 ethanoic acid molecules dissociate in water.
Note: The commercial name of ethanoic acid is acetic acid that is found in vinegar. Do not get confused if acetic acid is mentioned instead of ethanoic acid. Remember that it is not the alkyl group attached to the carboxylic acid group that has 2 carbons but the whole molecule that has 2 carbons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




