
Draw the structure of benzene and cyclohexane.
Answer
501.3k+ views
Hint: Cyclohexane is a cyclic ring with six carbon atoms. All the carbon atoms are saturated which means that they are $s{p^3}$ hybridised. Cyclohexane is a cycloalkane because a prefix “-ane” is present in its name. Benzene is also a ring with six carbon atoms but all the six carbon atoms are $s{p^2}$ hybridised. Benzene is an aromatic ring because it is planar, cyclic and has alternate double bonds. In the solution we will see the structures of benzene and cyclohexane.
Complete answer:
We know that the IUPAC name of the alkane ends with a prefix “-ane”. So, cyclohexane is an alkane and it is a cyclic ring with six carbon atoms. All the carbon atoms are saturated which means that they are $s{p^3}$ hybridised.
So, the structure of cyclohexane is given as:
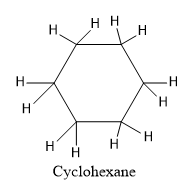
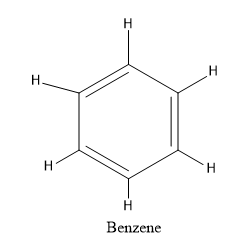
We know that Benzene is also a ring with six carbon atoms but all the six carbon atoms are $s{p^2}$ hybridised. Benzene is an aromatic ring. An aromatic ring has the characteristics such as it is planar, cyclic and has conjugation. Benzene rings satisfy all the conditions of aromaticity and it is aromatic in nature.
The structure of benzene is given as:
Note:
There are many conformations in which the cyclohexane exists such as chair form, boat form, twist boat form and half chair form. These conformations have different stability according to bond angles and energy. The stability order of these conformations is chair form> twist boat form> boat form> half chair form.
Complete answer:
We know that the IUPAC name of the alkane ends with a prefix “-ane”. So, cyclohexane is an alkane and it is a cyclic ring with six carbon atoms. All the carbon atoms are saturated which means that they are $s{p^3}$ hybridised.
So, the structure of cyclohexane is given as:

We know that Benzene is also a ring with six carbon atoms but all the six carbon atoms are $s{p^2}$ hybridised. Benzene is an aromatic ring. An aromatic ring has the characteristics such as it is planar, cyclic and has conjugation. Benzene rings satisfy all the conditions of aromaticity and it is aromatic in nature.
The structure of benzene is given as:

Note:
There are many conformations in which the cyclohexane exists such as chair form, boat form, twist boat form and half chair form. These conformations have different stability according to bond angles and energy. The stability order of these conformations is chair form> twist boat form> boat form> half chair form.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




