
Draw the structural formula of 2-chloropropane.
Answer
567k+ views
Hint: We can solve this problem by keeping in mind the IUPAC rules for nomenclature for the organic compound. For the IUPAC naming of compounds there are certain rules to be followed. The IUPAC nomenclature is a step wise rule to name the compound.
Complete Solution :
The IUPAC name of the given compound is 2-Chloropropane.
It is an aliphatic compound with the root word propane. Prop means three carbon chains which is the parent chain or the longest carbon chain and “ane” means presence of a single bond in the compound. 2-chloro means that the second position is occupied by the chlorine group.
Hence, the structure will be:
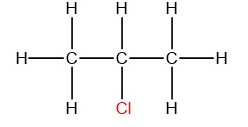
And, the structural formula will be ${{C}_{3}}{{H}_{7}}Cl$ .
Additional information:
IUPAC stands for international union for pure and applied chemistry, the steps to write the IUPAC nomenclature are mentioned below:
Step-1 select the longest carbon chain.
Step-2 identifies the substituents attached to the parent chain and number the parent chain from the end which gives the lowest number to the substituent.
- While writing the IUPAC nomenclature the substituents present in the given compound should be arranged in alphabetical order and if the same substituent occurs more than one then the prefix like di, tri, tetra etc. are used. It is important to put a comma between two numbers and a dash between a number and the name of the substituent. While arranging the substituents in alphabetical order we should ignore the prefix like di, tri, and tetra and arrange on the basis of the first letter of the substituent name.
Note: It is not important that every substituent should have a number and in case or aromatic compounds such as benzene position can also be referred as ortho, meta and para.
Complete Solution :
The IUPAC name of the given compound is 2-Chloropropane.
It is an aliphatic compound with the root word propane. Prop means three carbon chains which is the parent chain or the longest carbon chain and “ane” means presence of a single bond in the compound. 2-chloro means that the second position is occupied by the chlorine group.
Hence, the structure will be:

And, the structural formula will be ${{C}_{3}}{{H}_{7}}Cl$ .
Additional information:
IUPAC stands for international union for pure and applied chemistry, the steps to write the IUPAC nomenclature are mentioned below:
Step-1 select the longest carbon chain.
Step-2 identifies the substituents attached to the parent chain and number the parent chain from the end which gives the lowest number to the substituent.
- While writing the IUPAC nomenclature the substituents present in the given compound should be arranged in alphabetical order and if the same substituent occurs more than one then the prefix like di, tri, tetra etc. are used. It is important to put a comma between two numbers and a dash between a number and the name of the substituent. While arranging the substituents in alphabetical order we should ignore the prefix like di, tri, and tetra and arrange on the basis of the first letter of the substituent name.
Note: It is not important that every substituent should have a number and in case or aromatic compounds such as benzene position can also be referred as ortho, meta and para.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




